Lenacapavir: First Approval
- PMID: 36272024
- PMCID: PMC10267266
- DOI: 10.1007/s40265-022-01786-0
Lenacapavir: First Approval
Erratum in
-
Correction to: Lenacapavir: First Approval.Drugs. 2023 Jul;83(11):1061. doi: 10.1007/s40265-023-01908-2. Drugs. 2023. PMID: 37314634 Free PMC article. No abstract available.
Abstract
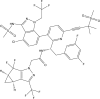
Lenacapavir (Sunlenca®) is a long-acting capsid inhibitor of human immunodeficiency virus type 1 (HIV-1) being developed by Gilead Sciences Inc. It is available as an oral tablet and injectable solution, with the latter being a slow-release formulation to allow bi-annual subcutaneous administration. In August 2022, lenacapavir received its first approval in the EU for use in combination with other antiretroviral(s) in adults with multi-drug resistant HIV infection, for whom it is otherwise not possible to construct a suppressive anti-viral regimen. This article summarizes the milestones in the development of lenacapavir leading to this first approval for the treatment of HIV-1 infection.
© 2022. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Julia Paik is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Figures
References
-
- Gilead Sciences Inc. Lenacapavir (Sunlenca®): EU summary of product characteristics. 2022. https://ema.europa.eu/. Accessed 21 Sep 2022.
-
- Gilead Sciences Inc. Gilead announces first regulatory approval for Sunlenca® (lenacapavir), the only twice-yearly HIV treatment option [media release]. 22 Aug 2022. https://www.gilead.com/.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical