Neurochemical correlates of synapse density in a Huntington's disease mouse model
- PMID: 36272099
- PMCID: PMC9892354
- DOI: 10.1111/jnc.15714
Neurochemical correlates of synapse density in a Huntington's disease mouse model
Abstract
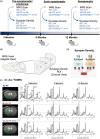
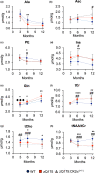
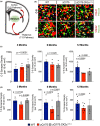
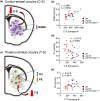
Striatal medium spiny neurons are highly susceptible in Huntington's disease (HD), resulting in progressive synaptic perturbations that lead to neuronal dysfunction and death. Non-invasive imaging techniques, such as proton magnetic resonance spectroscopy (1 H-MRS), are used in HD mouse models and patients with HD to monitor neurochemical changes associated with neuronal health. However, the association between brain neurochemical alterations and synaptic dysregulation remains unknown, limiting our ability to monitor potential treatments that may affect synapse function. We conducted in vivo longitudinal 1 H-MRS in the striatum followed by ex vivo analyses of excitatory synapse density of two synaptic circuits disrupted in HD, thalamo-striatal (T-S), and cortico-striatal (C-S) pathways, to assess the relationship between neurochemical alterations and changes in synapse density. We used the zQ175(Tg/0) HD mouse model as well as zQ175 mice lacking one allele of CK2α'(zQ175(Tg/0) :CK2α'(+/-) ), a kinase previously shown to regulate synapse function in HD. Longitudinal analyses of excitatory synapse density showed early and sustained reduction in T-S synapses in zQ175 mice, preceding C-S synapse depletion, which was rescued in zQ175:CK2α'(+/-) . Changes in T-S and C-S synapses were accompanied by progressive alterations in numerous neurochemicals between WT and HD mice. Linear regression analyses showed C-S synapse number positively correlated with 1 H-MRS-measured levels of GABA, while T-S synapse number positively correlated with levels of phosphoethanolamine and negatively correlated with total creatine levels. These associations suggest that these neurochemical concentrations measured by 1 H-MRS may facilitate monitoring circuit-specific synaptic dysfunction in the zQ175 mouse model and in other HD pre-clinical studies.
Keywords: 1H-MRS; CK2 alpha prime; Huntington's disease; neurochemicals; synapse density; zQ175.
© 2022 The Authors. Journal of Neurochemistry published by John Wiley & Sons Ltd on behalf of International Society for Neurochemistry.
Conflict of interest statement
Dr. Öz consults for IXICO Technologies Limited and uniQure biopharma B.V., serves on the Scientific Advisory Board of BrainSpec Inc. and receives research support from Biogen.
Figures
References
-
- Agarwal, S. , Schaefer, M. L. , Krall, C. , & Johns, R. A. (2022). Isoflurane disrupts postsynaptic density‐95 protein interactions causing neuronal synapse loss and cognitive impairment in juvenile mice via canonical NO‐mediated protein Kinase‐G signaling. Anesthesiology, 137, 212–231. - PMC - PubMed
-
- Behrens, P. F. , Franz, P. , Woodman, B. , Lindenberg, K. S. , & Landwehrmeyer, G. B. (2002). Impaired glutamate transport and glutamate–glutamine cycling: downstream effects of the Huntington mutation. Brain, 125, 1908–1922. - PubMed
-
- Deng, Y. P. , Wong, T. , Wan, J. Y. , & Reiner, A. (2014). Differential loss of thalamostriatal and corticostriatal input to striatal projection neuron types prior to overt motor symptoms in the Q140 knock‐in mouse model of Huntington's disease. Frontiers in Systems Neuroscience, 8, 198. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

