G9a Modulates Lipid Metabolism in CD4 T Cells to Regulate Intestinal Inflammation
- PMID: 36272457
- PMCID: PMC9892272
- DOI: 10.1053/j.gastro.2022.10.011
G9a Modulates Lipid Metabolism in CD4 T Cells to Regulate Intestinal Inflammation
Abstract
Background & aims: Although T-cell intrinsic expression of G9a has been associated with murine intestinal inflammation, mechanistic insight into the role of this methyltransferase in human T-cell differentiation is ill defined, and manipulation of G9a function for therapeutic use against inflammatory disorders is unexplored.
Methods: Human naive T cells were isolated from peripheral blood and differentiated in vitro in the presence of a G9a inhibitor (UNC0642) before being characterized via the transcriptome (RNA sequencing), chromatin accessibility (assay for transposase-accessible chromatin by sequencing), protein expression (cytometry by time of flight, flow cytometry), metabolism (mitochondrial stress test, ultrahigh performance liquid chromatography-tandem mas spectroscopy) and function (T-cell suppression assay). The in vivo role of G9a was assessed using 3 murine models.
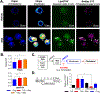
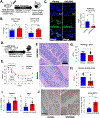
Results: We discovered that pharmacologic inhibition of G9a enzymatic function in human CD4 T cells led to spontaneous generation of FOXP3+ T cells (G9a-inibitors-T regulatory cells [Tregs]) in vitro that faithfully reproduce human Tregs, functionally and phenotypically. Mechanistically, G9a inhibition altered the transcriptional regulation of genes involved in lipid biosynthesis in T cells, resulting in increased intracellular cholesterol. Metabolomic profiling of G9a-inibitors-Tregs confirmed elevated lipid pathways that support Treg development through oxidative phosphorylation and enhanced lipid membrane composition. Pharmacologic G9a inhibition promoted Treg expansion in vivo upon antigen (gliadin) stimulation and ameliorated acute trinitrobenzene sulfonic acid-induced colitis secondary to tissue-specific Treg development. Finally, Tregs lacking G9a expression (G9a-knockout Tregs) remain functional chronically and can rescue T-cell transfer-induced colitis.
Conclusion: G9a inhibition promotes cholesterol metabolism in T cells, favoring a metabolic profile that facilitates Treg development in vitro and in vivo. Our data support the potential use of G9a inhibitors in the treatment of immune-mediated conditions including inflammatory bowel disease.
Keywords: Cholesterol; Inflammatory Bowel Disease; Lipid Metabolism; Regulatory T Cells.
Copyright © 2023 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of interest
The authors disclose no conflicts.
Figures





References
-
- Smids C, Horjus Talabur Horje CS, Drylewicz J, et al. Intestinal T cell profiling in inflammatory bowel disease: linking T cell subsets to disease activity and disease course. J Crohns Colitis 2018;12:465–475. - PubMed
-
- Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol 2005;6:345–352. - PubMed
-
- Ohkura N, Kitagawa Y, Sakaguchi S. Development and maintenance of regulatory T cells. Immunity 2013; 38:414–423. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

