TRPM8 contributes to sex dimorphism by promoting recovery of normal sensitivity in a mouse model of chronic migraine
- PMID: 36272975
- PMCID: PMC9588003
- DOI: 10.1038/s41467-022-33835-3
TRPM8 contributes to sex dimorphism by promoting recovery of normal sensitivity in a mouse model of chronic migraine
Abstract
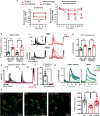
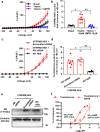
TRPA1 and TRPM8 are transient receptor potential channels expressed in trigeminal neurons that are related to pathophysiology in migraine models. Here we use a mouse model of nitroglycerine-induced chronic migraine that displays a sexually dimorphic phenotype, characterized by mechanical hypersensitivity that develops in males and females, and is persistent up to day 20 in female mice, but disappears by day 18 in male mice. TRPA1 is required for development of hypersensitivity in males and females, whereas TRPM8 contributes to the faster recovery from hypersensitivity in males. TRPM8-mediated antinociception effects required the presence of endogenous testosterone in males. Administration of exogenous testosterone to females and orchidectomized males led to recovery from hypersensitivity. Calcium imaging and electrophysiological recordings in in vitro systems confirmed testosterone activity on murine and human TRPM8, independent of androgen receptor expression. Our findings suggest a protective function of TRPM8 in shortening the time frame of hypersensitivity in a mouse model of migraine.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

