In silico evaluation of WHO-endorsed molecular methods to detect drug resistant tuberculosis
- PMID: 36273016
- PMCID: PMC9587982
- DOI: 10.1038/s41598-022-21025-6
In silico evaluation of WHO-endorsed molecular methods to detect drug resistant tuberculosis
Abstract
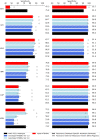
Universal drug susceptibility testing (DST) for tuberculosis is a major goal of the END TB strategy. PCR-based molecular diagnostic tests have been instrumental in increasing DST globally and several assays have now been endorsed by the World Health Organization (WHO) for use in the diagnosis of drug resistance. These endorsed assays, however, each interrogate a limited number of mutations associated with resistance, potentially limiting their sensitivity compared to sequencing-based methods. We applied an in silico method to compare the sensitivity and specificity of WHO-endorsed molecular based diagnostics to the mutation set identified by the WHO mutations catalogue using phenotypic DST as the reference. We found that, in silico, the mutation sets used by probe-based molecular diagnostic tests to identify rifampicin, isoniazid, pyrazinamide, levofloxacin, moxifloxacin, amikacin, capreomycin and kanamycin resistance produced similar sensitivities and specificities to the WHO mutation catalogue. PCR-based diagnostic tests were most sensitive for drugs where mechanisms of resistance are well established and localised to small genetic regions or a few prevalent mutations. Approaches using sequencing technologies can provide advantages for drugs where our knowledge of resistance is limited, or where complex resistance signatures exist.
© 2022. The Author(s).
Conflict of interest statement
A.B., M.S., S.B.G., S.U., A.S., R.E.C. are consultants or employees of FIND, the global alliance for diagnostics. T.M.W. declares no competing interests.
Figures

References
-
- World Health Organization (WHO) Global Tuberculosis Report 2021. WHO; 2021.
-
- World Health Organization (WHO). The End TB Strategy: Global Strategy and Targets for Tuberculosis Prevention, Care and Control After 2015. (2015). http://www.who.int/tb/strategy/End_TB_Strategy.pdf?ua=1.
-
- World Health Organization (WHO). Implementing the End TB Strategy: The Essentials (2015). https://www.who.int/tb/publications/2015/end_tb_essential.pdf.
-
- Organization, W. H. Technical Manual for Drug Susceptibility Testing of Medicines Used in the Treatment of Tuberculosis. Report No. 9789241514842. (World Health Organization, 2018).

