Singleplex, multiplex and pooled sample real-time RT-PCR assays for detection of SARS-CoV-2 in an occupational medicine setting
- PMID: 36273023
- PMCID: PMC9587995
- DOI: 10.1038/s41598-022-22106-2
Singleplex, multiplex and pooled sample real-time RT-PCR assays for detection of SARS-CoV-2 in an occupational medicine setting
Abstract
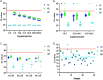
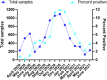
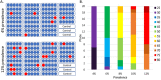
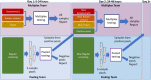
For workplaces which cannot operate as telework or remotely, there is a critical need for routine occupational SARS-CoV-2 diagnostic testing. Although diagnostic tests including the CDC 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel (CDC Diagnostic Panel) (EUA200001) were made available early in the pandemic, resource scarcity and high demand for reagents and equipment necessitated priority of symptomatic patients. There is a clearly defined need for flexible testing methodologies and strategies with rapid turnaround of results for (1) symptomatic, (2) asymptomatic with high-risk exposures and (3) asymptomatic populations without preexisting conditions for routine screening to address the needs of an on-site work force. We developed a distinct SARS-CoV-2 diagnostic assay based on the original CDC Diagnostic Panel (EUA200001), yet, with minimum overlap for currently employed reagents to eliminate direct competition for limited resources. As the pandemic progressed with testing loads increasing, we modified the assay to include 5-sample pooling and amplicon target multiplexing. Analytical sensitivity of the pooled and multiplexed assays was rigorously tested with contrived positive samples in realistic patient backgrounds. Assay performance was determined with clinical samples previously assessed with an FDA authorized assay. Throughout the pandemic we successfully tested symptomatic, known contact and travelers within our occupational population with a ~ 24-48-h turnaround time to limit the spread of COVID-19 in the workplace. Our singleplex assay had a detection limit of 31.25 copies per reaction. The three-color multiplexed assay maintained similar sensitivity to the singleplex assay, while tripling the throughput. The pooling assay further increased the throughput to five-fold the singleplex assay, albeit with a subtle loss of sensitivity. We subsequently developed a hybrid 'multiplex-pooled' strategy to testing to address the need for both rapid analysis of samples from personnel at high risk of COVID infection and routine screening. Herein, our SARS-CoV-2 assays specifically address the needs of occupational healthcare for both rapid analysis of personnel at high-risk of infection and routine screening that is essential for controlling COVID-19 disease transmission. In addition to SARS-CoV-2 and COVID-19, this work demonstrates successful flexible assays developments and deployments with implications for emerging highly transmissible diseases and future pandemics.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, Spitters C, Ericson K, Wilkerson S, Tural A, Diaz G, Cohn A, Fox L, Patel A, Lu X, Lindstrom S, Pallansch MA, Weldon WC, Biggs HM, Uyeki TM, Pillai SK. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. - DOI - PMC - PubMed
-
- Moreland, A., Herlihy, C., Tynan, M. A., Sunshine, G., McCord, R. F., Hilton, C., Poovey, J., Werner, A. K., Jones, C. D., Fulmer, E. B., Gundlapalli, A. V., Strosnider, H., Potvien, A., Garcia, M. C., Honeycutt, S., & Baldwin, G. Timing of State and Territorial COVID-19 Stay-at-Home Orders and Changes in Population Movement—United States, March 1–May 31, 2020; US Department of Health and Human Services/Centers for Disease Control and Prevention: 2020; pp 1198–1203.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

