Vitamin D enhances type I IFN signaling in COVID-19 patients
- PMID: 36273032
- PMCID: PMC9588043
- DOI: 10.1038/s41598-022-22307-9
Vitamin D enhances type I IFN signaling in COVID-19 patients
Abstract
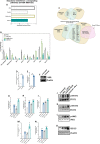
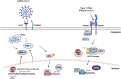
The ability of Vitamin D (VitD) to modulate antiviral responses through induction of antimicrobial peptide is well established. However, the effect of VitD on host responses to SARS-CoV-2 is not well investigated. We here report the ability of VitD to enhance host IFN-alpha/beta (a/β) signaling both in vitro and among severe COVID-19 patients treated with VitD. Blood and saliva specimens were obtained from severe COVID-19 patients treated (43 patients), or not (37 patients), with vitD, during their stay in intensive care unit. Patients were followed up to 29 days following admission, and patient survival outcomes were collected. Higher activity levels of RIG-1/MDA-5 and JAK-STAT signaling pathways were observed with significantly higher gene and protein levels of antiviral interferon stimulating genes (ISGs) such as MX-1 and ISG-15; both in vitro, following treatment of PBMCs with vitD, and in whole blood and saliva specimens of VitD treated patients. Moreover, VitD treated patients had lower risk of all-cause mortality by day 29 compared to untreated patients (adjusted hazard ratio, 0.37, 95% confidence interval of 0.14-0.94; P = 0.038). The herein uncovered regulatory role of VitD on type I IFNs suggests the importance of insuring a normal level of VitD for the prevention and probably treatment of SARS-CoV-2 infection. Additional mechanistic studies, however, are needed to fully elucidate the antiviral effects of VitD particularly in the setting of COVID-19 infection.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

