A clinical review of HIV integrase strand transfer inhibitors (INSTIs) for the prevention and treatment of HIV-1 infection
- PMID: 36273165
- PMCID: PMC9588231
- DOI: 10.1186/s12977-022-00608-1
A clinical review of HIV integrase strand transfer inhibitors (INSTIs) for the prevention and treatment of HIV-1 infection
Abstract
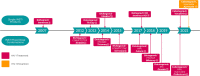
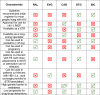
Integrase strand transfer inhibitors (INSTIs) have improved the treatment of human immunodeficiency virus (HIV). There are currently four approved for use in treatment-naïve individuals living with HIV; these include first generation raltegravir, elvitegravir, and second generation dolutegravir and bictegravir. The most recent INSTI, cabotegravir, is approved for (1) treatment of HIV infection in adults to replace current antiretroviral therapy in individuals who maintain virologic suppression on a stable antiretroviral regimen without history of treatment failure and no known resistance to its components and (2) pre-exposure prophylaxis in individuals at risk of acquiring HIV-1 infection. Cabotegravir can be administered intramuscularly as a monthly or bi-monthly injection depending on the indication. This long-acting combination has been associated with treatment satisfaction in clinical studies and may be helpful for individuals who have difficulty taking daily oral medications. Worldwide, second generation INSTIs are preferred for treatment-naïve individuals. Advantages of these INSTIs include their high genetic barrier to resistance, limited drug-drug interactions, excellent rates of virologic suppression, and favorable tolerability. Few INSTI resistance-associated mutations have been reported in clinical trials involving dolutegravir, bictegravir and cabotegravir. Other advantages of specific INSTIs include their use in various populations such as infants and children, acute HIV infection, and individuals of childbearing potential. The most common adverse events observed in clinical studies involving INSTIs included diarrhea, nausea, insomnia, fatigue, and headache, with very low rates of treatment discontinuation versus comparator groups. The long-term clinical implications of weight gain associated with second generation INSTIs dolutegravir and bictegravir warrants further study. This review summarizes key clinical considerations of INSTIs in terms of clinical pharmacology, drug-drug interactions, resistance, and provides perspective on clinical decision-making. Additionally, we summarize major clinical trials evaluating the efficacy and safety of INSTIs in treatment-naïve patients living with HIV as well as individuals at risk of acquiring HIV infection.
Keywords: Bictegravir; Cabotegravir; Dolutegravir; Elvitegravir; HIV; Integrase strand transfer inhibitor (INSTI); Pre-exposure prophylaxis; Raltegravir; Treatment naïve.
© 2022. The Author(s).
Conflict of interest statement
ACM currently works for ViiV Healthcare as a Medical Science Liaison and RCG currently works for GlaxoSmithKline as an HIV Medical Information Scientist.
Figures
References
-
- WHO Guidelines Approved by the Guidelines Review Committee . In Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach. Geneva: World Health Organization; 2021. - PubMed
-
- Guidelines for the use of antiretroviral agents in adults and adolescents with HIV [https://clinicalinfo.hiv.gov/sites/default/files/inline-files/AdultandAd...]
-
- EACS Guidelines version 11.0. October 2021.
-
- Molina JM, Clotet B, van Lunzen J, Lazzarin A, Cavassini M, Henry K, Kulagin V, Givens N, Brennan C, de Oliveira CF. Once-daily dolutegravir is superior to once-daily darunavir/ritonavir in treatment-naive HIV-1-positive individuals: 96 week results from FLAMINGO. J Int AIDS Soc. 2014;17:19490. doi: 10.7448/IAS.17.4.19490. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

