Tetrandrine ameliorates cognitive deficits and mitigates tau aggregation in cell and animal models of tauopathies
- PMID: 36273169
- PMCID: PMC9587578
- DOI: 10.1186/s12929-022-00871-6
Tetrandrine ameliorates cognitive deficits and mitigates tau aggregation in cell and animal models of tauopathies
Abstract
Background: Tauopathies are neurodegenerative diseases that are associated with the pathological accumulation of tau-containing tangles in the brain. Tauopathy can impair cognitive and motor functions and has been observed in Alzheimer's disease (AD) and frontotemporal dementia (FTD). The aetiology of tauopathy remains mysterious; however, recent studies suggest that the autophagic-endolysosomal function plays an essential role in the degradation and transmission of pathological tau. We previously demonstrated that tetrandrine could ameliorate memory functions and clear amyloid plaques in transgenic AD mice by restoring autophagic-endolysosomal function. However, the efficacy of tetrandrine and the associated therapeutic mechanism in tauopathies have not been evaluated and elucidated.
Methods: Novel object recognition, fear conditioning and electrophysiology were used to evaluate the effects of tetrandrine on memory functions in transgenic tau mice. Western blotting and immunofluorescence staining were employed to determine the effect of tetrandrine on autophagy and tau clearance in vivo. Calcium (Ca2+) imaging and flow cytometry were used to delineate the role of pathological tau and tetrandrine in lysosomal Ca2+ and pH homeostasis. Biochemical BiFC fluorescence, Western blotting and immunofluorescence staining were used to evaluate degradation of hyperphosphorylated tau in vitro, whereas coculture of brain slices with isolated microglia was used to evaluate tau clearance ex vivo.
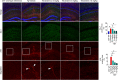
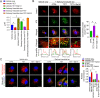
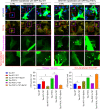
Results: We observed that tetrandrine treatment mitigated tau tangle development and corrected memory impairment in Thy1-hTau.P301S transgenic mice. Mechanistically, we showed that mutant tau expression disrupts lysosome pH by increasing two-pore channel 2 (TPC2)-mediated Ca2+ release, thereby contributing to lysosome alkalinization. Tetrandrine inhibits TPC2, thereby restoring the lysosomal pH, promotes tau degradation via autophagy, and ameliorates tau aggregation. Furthermore, in an ex vivo assay, we demonstrated that tetrandrine treatment promotes pathological tau clearance by microglia.
Conclusions: Together, these findings suggest that pathological tau disturbs endolysosomal homeostasis to impair tau clearance. This impairment results in a vicious cycle that accelerates disease pathogenesis. The success of tetrandrine in reducing tau aggregation suggests first, that tetrandrine could be an effective drug for tauopathies and second, that rescuing lysosomal Ca2+ homeostasis, thereby restoring ALP function, could be an effective general strategy for the development of novel therapies for tauopathies.
Keywords: Autophagy; Calcium dysregulation; Lysosome; Tauopathy; Tetrandrine; Two-pore channel 2.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- As A. 2017 Alzheimer's disease facts and figures. Alzheimers Dement. 2017;13(4):325–373.
-
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. - PubMed
-
- Xin S-H, Tan L, Cao X, Yu J-T, Tan L. Clearance of amyloid beta and tau in Alzheimer’s disease: from mechanisms to therapy. Neurotox Res. 2018;34(3):733–748. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

