Proton FLASH Radiotherapy for the Treatment of Symptomatic Bone Metastases: The FAST-01 Nonrandomized Trial
- PMID: 36273324
- PMCID: PMC9589460
- DOI: 10.1001/jamaoncol.2022.5843
Proton FLASH Radiotherapy for the Treatment of Symptomatic Bone Metastases: The FAST-01 Nonrandomized Trial
Erratum in
-
Change of Article Status to Open Access.JAMA Oncol. 2023 May 1;9(5):728. doi: 10.1001/jamaoncol.2023.0218. JAMA Oncol. 2023. PMID: 36862393 Free PMC article. No abstract available.
Abstract
Importance: To our knowledge, there have been no clinical trials of ultra-high-dose-rate radiotherapy delivered at more than 40 Gy/sec, known as FLASH therapy, nor first-in-human use of proton FLASH.
Objectives: To assess the clinical workflow feasibility and treatment-related toxic effects of FLASH and pain relief at the treatment sites.
Design, setting, and participants: In the FAST-01 nonrandomized trial, participants treated at Cincinnati Children's/UC Health Proton Therapy Center underwent palliative FLASH radiotherapy to extremity bone metastases. Patients 18 years and older with 1 to 3 painful extremity bone metastases and life expectancies of 2 months or more were eligible. Patients were excluded if they had foot, hand, and wrist metastases; metastases locally treated in the 2 weeks prior; metal implants in the treatment field; known enhanced tissue radiosensitivity; and implanted devices at risk of malfunction with radiotherapy. One of 11 patients who consented was excluded based on eligibility. The end points were evaluated at 3 months posttreatment, and patients were followed up through death or loss to follow-up for toxic effects and pain assessments. Of the 10 included patients, 2 died after the 2-month follow-up but before the 3-month follow-up; 8 participants completed the 3-month evaluation. Data were collected from November 3, 2020, to January 28, 2022, and analyzed from January 28, 2022, to September 1, 2022.
Interventions: Bone metastases were treated on a FLASH-enabled (≥40 Gy/sec) proton radiotherapy system using a single-transmission proton beam. This is consistent with standard of care using the same prescription (8 Gy in a single fraction) but on a conventional-dose-rate (approximately 0.03 Gy/sec) photon radiotherapy system.
Main outcome and measures: Main outcomes included patient time on the treatment couch, device-related treatment delays, adverse events related to FLASH, patient-reported pain scores, and analgesic use.
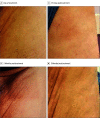
Results: A total of 10 patients (age range, 27-81 years [median age, 63 years]; 5 [50%] male) underwent FLASH radiotherapy at 12 metastatic sites. There were no FLASH-related technical issues or delays. The average (range) time on the treatment couch was 18.9 (11-33) minutes per patient and 15.8 (11-22) minutes per treatment site. Median (range) follow-up was 4.8 (2.3-13.0) months. Adverse events were mild and consistent with conventional radiotherapy. Transient pain flares occurred in 4 of the 12 treated sites (33%). In 8 of the 12 sites (67%) patients reported pain relief, and in 6 of the 12 sites (50%) patients reported a complete response (no pain).
Conclusions and relevance: In this nonrandomized trial, clinical workflow metrics, treatment efficacy, and safety data demonstrated that ultra-high-dose-rate proton FLASH radiotherapy was clinically feasible. The treatment efficacy and the profile of adverse events were comparable with those of standard-of-care radiotherapy. These findings support the further exploration of FLASH radiotherapy in patients with cancer.
Trial registration: ClinicalTrials.gov Identifier: NCT04592887.
Conflict of interest statement
Figures


Comment in
-
The First FLASH Clinical Trial-The Journey of a Thousand Miles Begins With 1 Step.JAMA Oncol. 2023 Jan 1;9(1):69-70. doi: 10.1001/jamaoncol.2022.5842. JAMA Oncol. 2023. PMID: 36273321 No abstract available.
-
FLASH Radiotherapy in a Value-Based Health Care Environment.JAMA Oncol. 2023 May 1;9(5):726-727. doi: 10.1001/jamaoncol.2023.0131. JAMA Oncol. 2023. PMID: 36951857 No abstract available.
References
-
- Loo JB, Schuler E, Lartey F, et al. . Delivery of ultra-rapid flash radiation therapy and demonstartion of normal tissue sparing after abdominal irradiation of mice. Int J Radiat Oncol Biol Phys. 2017;98(2)(suppl):E16. doi:10.1016/j.ijrobp.2017.02.101 - DOI

