Epigenetic upregulation of Schlafen11 renders WNT- and SHH-activated medulloblastomas sensitive to cisplatin
- PMID: 36273330
- PMCID: PMC10158119
- DOI: 10.1093/neuonc/noac243
Epigenetic upregulation of Schlafen11 renders WNT- and SHH-activated medulloblastomas sensitive to cisplatin
Abstract
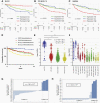
Background: Intensive chemotherapeutic regimens with craniospinal irradiation have greatly improved survival in medulloblastoma patients. However, survival markedly differs among molecular subgroups and their biomarkers are unknown. Through unbiased screening, we found Schlafen family member 11 (SLFN11), which is known to improve response to DNA damaging agents in various cancers, to be one of the top prognostic markers in medulloblastomas. Hence, we explored the expression and functions of SLFN11 in medulloblastoma.
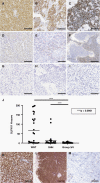
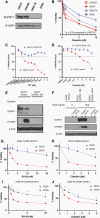
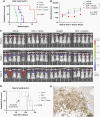
Methods: SLFN11 expression for each subgroup was assessed by immunohistochemistry in 98 medulloblastoma patient samples and by analyzing transcriptomic databases. We genetically or epigenetically modulated SLFN11 expression in medulloblastoma cell lines and determined cytotoxic response to the DNA damaging agents cisplatin and topoisomerase I inhibitor SN-38 in vitro and in vivo.
Results: High SLFN11 expressing cases exhibited significantly longer survival than low expressing cases. SLFN11 was highly expressed in the WNT-activated subgroup and in a proportion of the SHH-activated subgroup. While WNT activation was not a direct cause of the high expression of SLFN11, a specific hypomethylation locus on the SLFN11 promoter was significantly correlated with high SLFN11 expression. Overexpression or deletion of SLFN11 made medulloblastoma cells sensitive and resistant to cisplatin and SN-38, respectively. Pharmacological upregulation of SLFN11 by the brain-penetrant histone deacetylase-inhibitor RG2833 markedly increased sensitivity to cisplatin and SN-38 in SLFN11-negative medulloblastoma cells. Intracranial xenograft studies also showed marked sensitivity to cisplatin by SLFN11-overexpression in medulloblastoma cells.
Conclusions: High SLFN11 expression is one factor which renders favorable outcomes in WNT-activated and a subset of SHH-activated medulloblastoma possibly through enhancing response to cisplatin.
Keywords: DNA damaging agent; SLFN11; medulloblastoma.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Gajjar A, Chintagumpala M, Ashley D, et al. . Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7(10):813–820. - PubMed
-
- Packer RJ, Gajjar A, Vezina G, et al. . Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24(25):4202–4208. - PubMed

