Tocilizumab versus baricitinib in hospitalized patients with severe COVID-19: an open label, randomized controlled trial
- PMID: 36273769
- PMCID: PMC9636985
- DOI: 10.1016/j.cmi.2022.10.015
Tocilizumab versus baricitinib in hospitalized patients with severe COVID-19: an open label, randomized controlled trial
Abstract
Objective: Randomized controlled trials comparing tocilizumab and baricitinib in patients with coronavirus disease 2019 (COVID-19) are needed. This was an open-label, randomized controlled trial aiming to address this unmet need.
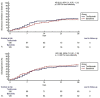
Methods: To determine whether baricitinib was non-inferior to tocilizumab, we assessed whether the upper boundary of the two-sided 95% CI of the hazard ratio (HR) did not exceed 1.50. The primary outcome was mechanical ventilation or death by day 28. Secondary outcomes included time to hospital discharge by day 28 and change in WHO progression scale at day 10.
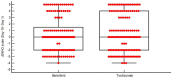
Results: We assigned 251 patients with COVID-19 and a PaO2/FiO2 ratio of <200 to receive either tocilizumab (n = 126) or baricitinib (n = 125) plus standard of care. Baricitinib was non-inferior to tocilizumab for the primary composite outcome of mechanical ventilation or death by day 28 (mechanical ventilation or death for patients who received baricitinib, 39.2% [n = 49/125]; mechanical ventilation or death for patients who received tocilizumab, 44.4% [n = 56/126]; HR, 0.83; 95% CI, 0.56-1.21; p 0.001 for non-inferiority). Baricitinib was non-inferior to tocilizumab for the time to hospital discharge within 28 days (patients who received baricitinib- discharged alive: 58.4% [n = 73/125] vs. patients who received tocilizumab- discharged alive: 52.4% [n = 66/126]; HR, 0.85; 95% CI, 0.61-1.18; p < 0.001 for non-inferiority). There was no significant difference between the baricitinib and tocilizumab arms in the change in WHO scale at day 10 (0.0 [95% CI, 0.0-0.0] vs. 0.0 [95% CI, 0.0-1.0]; p 0.83).
Discussion: In the setting of this trial, baricitinib was non-inferior to tocilizumab with regards to the composite outcome of mechanical ventilation or death by day 28 and the time to discharge by day 28 in patients with severe COVID-19.
Keywords: Baricitinib COVID-19; Mortality; PaO2/FiO2; Tocilizumab.
Copyright © 2022 European Society of Clinical Microbiology and Infectious Diseases. Published by Elsevier Ltd. All rights reserved.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

