The HD Reaction of Nitrogenase: a Detailed Mechanism
- PMID: 36274057
- PMCID: PMC10099629
- DOI: 10.1002/chem.202202502
The HD Reaction of Nitrogenase: a Detailed Mechanism
Abstract
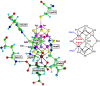
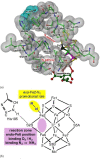
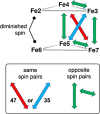
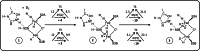
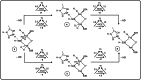
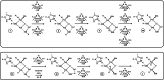
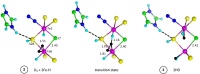
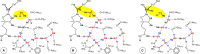
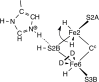
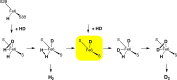
Nitrogenase is the enzyme that converts N2 to NH3 under ambient conditions. The chemical mechanism of this catalysis at the active site FeMo-co [Fe7 S9 CMo(homocitrate)] is unknown. An obligatory co-product is H2 , while exogenous H2 is a competitive inhibitor. Isotopic substitution using exogenous D2 revealed the N2 -dependent reaction D2 +2H+ +2e- →2HD (the 'HD reaction'), together with a collection of additional experimental characteristics and requirements. This paper describes a detailed mechanism for the HD reaction, developed and elaborated using density functional simulations with a 486-atom model of the active site and surrounding protein. First D2 binds at one Fe atom (endo-Fe6 coordination position), where it is flanked by H-Fe6 (exo position) and H-Fe2 (endo position). Then there is synchronous transfer of these two H atoms to bound D2 , forming one HD bound to Fe2 and a second HD bound to Fe6. These two HD dissociate sequentially. The final phase is recovery of the two flanking H atoms. These H atoms are generated, sequentially, by translocation of a proton from the protein surface to S3B of FeMo-co and combination with introduced electrons. The first H atom migrates from S3B to exo-Fe6 and the second from S3B to endo-Fe2. Reaction energies and kinetic barriers are reported for all steps. This mechanism accounts for the experimental data: (a) stoichiometry; (b) the N2 -dependence results from promotional N2 bound at exo-Fe2; (c) different N2 binding Km for the HD reaction and the NH3 formation reaction results from involvement of two different sites; (d) inhibition by CO; (e) the non-occurrence of 2HD→H2 +D2 results from the synchronicity of the two transfers of H to D2 ; (f) inhibition of HD production at high pN2 is by competitive binding of N2 at endo-Fe6; (g) the non-leakage of D to solvent follows from the hydrophobic environment and irreversibility of proton introduction.
Keywords: HD reaction; density functional calculations; enzyme catalysis; nitrogenases; reaction mechanisms.
© 2022 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The author declares no conflict of interest.
Figures
Similar articles
-
Calculating the chemical mechanism of nitrogenase: new working hypotheses.Dalton Trans. 2022 Aug 23;51(33):12717-12728. doi: 10.1039/d2dt01920e. Dalton Trans. 2022. PMID: 35946501
-
The binding of reducible N2 in the reaction domain of nitrogenase.Dalton Trans. 2023 Feb 14;52(7):2013-2026. doi: 10.1039/d2dt03599e. Dalton Trans. 2023. PMID: 36691966
-
The hydrogen chemistry of the FeMo-co active site of nitrogenase.J Am Chem Soc. 2005 Aug 10;127(31):10925-42. doi: 10.1021/ja0504946. J Am Chem Soc. 2005. PMID: 16076199
-
Computational Investigations of the Chemical Mechanism of the Enzyme Nitrogenase.Chembiochem. 2020 Jun 15;21(12):1671-1709. doi: 10.1002/cbic.201900636. Epub 2020 Jan 21. Chembiochem. 2020. PMID: 31803989 Review.
-
Elucidating the coordination chemistry and mechanism of biological nitrogen fixation.Chem Asian J. 2007 Aug 3;2(8):936-46. doi: 10.1002/asia.200700131. Chem Asian J. 2007. PMID: 17614310 Review.
Cited by
-
Biomimetic [MFe3S4]3+ Cubanes (M = V/Mo) as Catalysts for a Fischer-Tropsch-like Hydrocarbon Synthesis─A Computational Study.Inorg Chem. 2025 Jan 13;64(1):479-494. doi: 10.1021/acs.inorgchem.4c04995. Epub 2024 Dec 27. Inorg Chem. 2025. PMID: 39727298 Free PMC article.
References
-
- None
-
- Burgess B. K., Lowe D. J., Chem. Rev. 1996, 96, 2983–3011; - PubMed
-
- Howard J. B., Rees D. C., Chem. Rev. 1996, 96, 2965–2982; - PubMed
-
- Christiansen J., Dean D. R., Seefeldt L. C., Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 269–295; - PubMed
-
- Igarashi R. Y., Seefeldt L. C., Crit. Rev. Biochem. Mol. Biol. 2003, 38, 351–384; - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

