American College of Rheumatology/EULAR Remission Criteria for Rheumatoid Arthritis: 2022 Revision
- PMID: 36274193
- PMCID: PMC10092655
- DOI: 10.1002/art.42347
American College of Rheumatology/EULAR Remission Criteria for Rheumatoid Arthritis: 2022 Revision
Abstract
Objective: In 2011, the American College of Rheumatology (ACR) and EULAR endorsed provisional criteria for remission in rheumatoid arthritis (RA), both Boolean- and index-based. Based on recent studies indicating that a higher threshold for the patient global assessment (PtGA) may improve agreement between the 2 sets of criteria, our goals were to externally validate a revision of the Boolean remission criteria using a higher PtGA threshold and to validate the provisionally endorsed index-based criteria.
Methods: We used data from 4 randomized trials comparing biologic disease-modifying antirheumatic drugs to methotrexate or placebo. We tested the higher proposed PtGA threshold of 2 cm (Boolean2.0) (range 0-10 cm) compared to the original threshold of 1 cm (Boolean1.0). We analyzed agreement between the Boolean- and index-based criteria (Simplified Disease Activity Index [SDAI] and Clinical Disease Activity Index [CDAI]) for remission and examined how well each remission definition predicted later good physical function (Health Assessment Questionnaire [HAQ] score ≤0.5) and radiographic nonprogression.
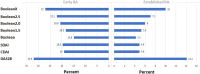
Results: Data from 2,048 trial participants, 1,101 with early RA and 947 with established RA, were included. The proportion of patients with disease in remission at 6 months after treatment initiation increased when using Boolean2.0 compared to Boolean1.0, from 14.8% to 20.6% in early RA and 4.2% to 6.0% in established RA. Agreement between Boolean2.0 and the SDAI or CDAI remission criteria was better than for Boolean1.0, particularly in early disease. Boolean2.0, SDAI, and CDAI remission criteria had similar positive likelihood ratios (LRs) to predict radiographic nonprogression and a HAQ score of ≤0.5 (positive LR 3.8-4.3). The omission of PtGA (BooleanX) worsened the prediction of good functional outcomes.
Conclusion: Using the Boolean 2.0 criteria classifies more patients as achieving remission and increases the agreement with index-based remission criteria without jeopardizing predictive value for radiographic or functional outcomes. This revised Boolean definition and the previously provisionally endorsed index-based criteria were endorsed by ACR and EULAR.
© 2022 The Authors. Arthritis & Rheumatology published by Wiley Periodicals LLC on behalf of American College of Rheumatology.
Figures

References
-
- Felson DT, Anderson JJ, Boers M, et al. The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. Arthritis Rheum 1993;36:729–40. - PubMed
-
- Scott D, van Riel P, van der Heijde D, et al. Assessing disease activity in rheumatoid arthritis: the EULAR handbook of standard methods. On behalf of the EULAR Standing Committee for International Clinical Studies Including Therapeutic Trials. Zürich: EULAR; 1993.
-
- Singh JA, Saag KG, Bridges SL Jr, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol 2016;68:1–26. - PubMed
-
- Smolen JS, Landewé R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease‐modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017;76:960–977. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

