Extracorporeal membrane oxygenation in COVID-19 compared to other etiologies of acute respiratory failure: A single-center experience
- PMID: 36274533
- PMCID: PMC9582301
- DOI: 10.1016/j.hrtlng.2022.10.003
Extracorporeal membrane oxygenation in COVID-19 compared to other etiologies of acute respiratory failure: A single-center experience
Abstract
Background: The COVID-19 pandemic has led to a boom in the use of V-V ECMO for ARDS secondary to COVID. Comparisons of outcomes of ECMO for COVID to ECMO for influenza have emerged. Very few comparisons of ECMO for COVID to ECMO for ARDS of all etiologies are available.
Objectives: To compare clinically important outcome measures in recipients of ECMO for COVID to those observed in recipients of ECMO for ARDS of other etiologies.
Methods: V-V ECMO recipients between March 2020 and March 2022 consisted exclusively of COVID patients and formed the COVID ECMO group. All patients who underwent V-V ECMO for ARDS between January 2014 and March 2020 were eligible for analysis as the non-COVID ECMO comparator group. The primary outcome was survival to hospital discharge. Secondary outcomes included ECMO decannulation, ECMO duration >30 days, and serious complications.
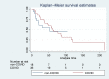
Results: Thirty-six patients comprised the COVID ECMO group and were compared to 18 non-COVID ECMO patients. Survival to hospital discharge was not significantly different between the two groups (33% in COVID vs. 50% in non-COVID; p = 0.255) nor was there a significant difference in the rate of non-palliative ECMO decannulation. The proportion of patients connected to ECMO for >30 days was significantly higher in the COVID ECMO group: 69% vs. 17%; p = 0.001. There was no significant difference in serious complications.
Conclusion: This study could not identify a statistically significant difference in hospital survival and rate of successful ECMO decannulation between COVID ECMO and non-COVID ECMO patients. Prolonged ECMO may be more common in COVID. Complications were not significantly different.
Keywords: Acute respiratory distress syndrome; COVID-19; Extracorporeal membrane oxygenation; SARS-CoV-2.
Copyright © 2022. Published by Elsevier Inc.
Conflict of interest statement
Declaration of Competing Interest None of the authors has any relevant competing interest to disclose.
Figures
References
-
- Ashbaugh D.G., Bigelow D.B., Petty T.L., Levine B.E. Acute respiratory distress in adults. Lancet. 1967;2:319–323. - PubMed
-
- Peek G.J., Mugford M., Tiruvoipati R., et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351–1363. [published correction appears in Lancet. 2009 Oct 17;374(9698):1330] - PubMed
-
- Bellani G., Laffey J.G., Pham T., et al. epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. [published correction appears in JAMA. 2016 Jul 19;316(3):350] [published correction appears in JAMA. 2016 Jul 19;316(3):350] - PubMed
-
- Combes A., Hajage D., Capellier G., et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378:1965–1975. - PubMed
-
- Goligher E.C., Tomlinson G., Hajage D., et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome and posterior probability of mortality benefit in a post hoc Bayesian analysis of a randomized clinical trial. JAMA. 2018;320:2251–2259. [published correction appears in JAMA. 2019 Jun 11;321(22):2245] - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous