Night vision restored in days after decades of congenital blindness
- PMID: 36274938
- PMCID: PMC9579015
- DOI: 10.1016/j.isci.2022.105274
Night vision restored in days after decades of congenital blindness
Abstract
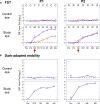
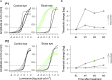
Signaling of vision to the brain starts with the retinal phototransduction cascade which converts visible light from the environment into chemical changes. Vision impairment results when mutations inactivate proteins of the phototransduction cascade. A severe monogenically inherited blindness, Leber congenital amaurosis (LCA), is caused by mutations in the GUCY2D gene, leading to a molecular defect in the production of cyclic GMP, the second messenger of phototransduction. We studied two patients with GUCY2D-LCA who were undergoing gene augmentation therapy. Both patients had large deficits in rod photoreceptor-based night vision before intervention. Within days of therapy, rod vision in both patients changed dramatically; improvements in visual function and functional vision in these hyper-responding patients reached more than 3 log10 units (1000-fold), nearing healthy rod vision. Quick activation of the complex molecular pathways from retinal photoreceptor to visual cortex and behavior is thus possible in patients even after being disabled and dormant for decades.
Keywords: Clinical genetics; Health sciences; Medicine.
© 2022 The Author(s).
Conflict of interest statement
S.E.B. and S.L.B. are scientific founders of and equity holders in Atsena Therapeutics, Inc. and are patent holders on the use of AAV-GUCY2D for the treatment of LCA1. C.N.K., D.Y., and K.P.F. are employees of Atsena Therapeutics, Inc. All other authors have no competing financial interests.
Figures




References
-
- Bouzia Z., Georgiou M., Hull S., Robson A.G., Fujinami K., Rotsos T., Pontikos N., Arno G., Webster A.R., Hardcastle A.J., et al. GUCY2D-associated Leber congenital amaurosis: a retrospective natural history study in preparation for trials of novel therapies. Am. J. Ophthalmol. 2020;210:59–70. doi: 10.1016/j.ajo.2019.10.019. - DOI - PMC - PubMed
-
- Boye S.E., Boye S.L., Pang J., Ryals R., Everhart D., Umino Y., Neeley A.W., Besharse J., Barlow R., Hauswirth W.W. Functional and behavioral restoration of vision by gene therapy in the guanylate cyclase-1 (GC1) knockout mouse. PLoS One. 2010;5:e11306. doi: 10.1371/journal.pone.0011306. - DOI - PMC - PubMed
-
- Boye S.L., Conlon T., Erger K., Ryals R., Neeley A., Cossette T., Pang J., Dyka F.M., Hauswirth W.W., Boye S.E. Long-term preservation of cone photoreceptors and restoration of cone function by gene therapy in the guanylate cyclase-1 knockout (GC1KO) mouse. Invest. Ophthalmol. Vis. Sci. 2011;52:7098–7108. doi: 10.1167/iovs.11-7867. - DOI - PMC - PubMed
-
- Boye S.L., Peshenko I.V., Huang W.C., Min S.H., McDoom I., Kay C.N., Liu X., Dyka F.M., Foster T.C., Umino Y., et al. AAV-mediated gene therapy in the guanylate cyclase (RetGC1/RetGC2) double knockout mouse model of Leber congenital amaurosis. Hum. Gene Ther. 2013;24:189–202. doi: 10.1089/hum.2012.193. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

