Hypocretin/orexin influences chronic sleep disruption injury in the hippocampus
- PMID: 36275002
- PMCID: PMC9582517
- DOI: 10.3389/fnagi.2022.1025402
Hypocretin/orexin influences chronic sleep disruption injury in the hippocampus
Abstract
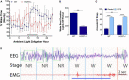
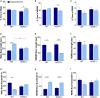
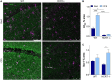
Chronic sleep disruption is a risk factor for Alzheimer's disease (AD), yet mechanisms by which sleep disturbances might promote or exacerbate AD are not understood. Short-term sleep loss acutely increases hippocampal amyloid β (Aβ) in wild type (WT) mice and long-term sleep loss increases amyloid plaque in AD transgenic mouse models. Both effects can be influenced by the wake-promoting neuropeptide, hypocretin (HCRT), but whether HCRT influences amyloid accumulation independent of sleep and wake timing modulation remains unclear. Here, we induced chronic fragmentation of sleep (CFS) in WT and HCRT-deficient mice to elicit similar arousal indices, sleep bout lengths and sleep bout numbers in both genotypes. We then examined the roles of HCRT in CFS-induced hippocampal Aβ accumulation and injury. CFS in WT mice resulted in increased Aβ42 in the hippocampus along with loss of cholinergic projections and loss of locus coeruleus neurons. Mice with HCRT deficiency conferred resistance to CFS Aβ42 accumulation and loss of cholinergic projections in the hippocampus yet evidenced similar CFS-induced loss of locus coeruleus neurons. Collectively, the findings demonstrate specific roles for orexin in sleep disruption hippocampal injury.
Significance statement: Chronic fragmentation of sleep (CFS) occurs in common conditions, including sleep apnea syndromes and chronic pain disorders, yet CFS can induce neural injury. Our results demonstrate that under conditions of sleep fragmentation, hypocretin/orexin is essential for the accumulation of amyloid-β and loss of cholinergic projections in the hippocampus observed in response to CFS yet does not influence locus coeruleus neuron response to CFS.
Keywords: amyloid; chronic sleep disruption; chronic sleep loss; degeneration; septohippocampal cholinergic system.
Copyright © 2022 Nick, Fenik, Zhu and Veasey.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Norepinephrine Drives Sleep Fragmentation Activation of Asparagine Endopeptidase, Locus Ceruleus Degeneration, and Hippocampal Amyloid-β42 Accumulation.J Neurosci. 2024 Jul 10;44(28):e1929232024. doi: 10.1523/JNEUROSCI.1929-23.2024. J Neurosci. 2024. PMID: 38830763 Free PMC article.
-
Transgenic Archaerhodopsin-3 Expression in Hypocretin/Orexin Neurons Engenders Cellular Dysfunction and Features of Type 2 Narcolepsy.J Neurosci. 2019 Nov 20;39(47):9435-9452. doi: 10.1523/JNEUROSCI.0311-19.2019. Epub 2019 Oct 18. J Neurosci. 2019. PMID: 31628177 Free PMC article.
-
Lack of hypocretin attenuates behavioral changes produced by glutamatergic activation of the perifornical-lateral hypothalamic area.Sleep. 2014 May 1;37(5):1011-20. doi: 10.5665/sleep.3680. Sleep. 2014. PMID: 24790280 Free PMC article.
-
The hypocretin/orexin system in sleep disorders: preclinical insights and clinical progress.Nat Sci Sleep. 2016 Mar 14;8:81-6. doi: 10.2147/NSS.S76711. eCollection 2016. Nat Sci Sleep. 2016. PMID: 27051324 Free PMC article. Review.
-
The sleep-wake cycle and Alzheimer's disease: what do we know?Neurodegener Dis Manag. 2014;4(5):351-62. doi: 10.2217/nmt.14.33. Neurodegener Dis Manag. 2014. PMID: 25405649 Free PMC article. Review.
Cited by
-
Relationship of Sleep Disorder with Neurodegenerative and Psychiatric Diseases: An Updated Review.Neurochem Res. 2024 Mar;49(3):568-582. doi: 10.1007/s11064-023-04086-5. Epub 2023 Dec 18. Neurochem Res. 2024. PMID: 38108952 Review.
-
Sex-dimorphic functions of orexin in neuropsychiatric disorders.Heliyon. 2024 Aug 15;10(16):e36402. doi: 10.1016/j.heliyon.2024.e36402. eCollection 2024 Aug 30. Heliyon. 2024. PMID: 39253145 Free PMC article. Review.
-
Management of sleep disturbance related to Alzheimer disease and dementia: An updated review of ClinicalTrials.gov.Medicine (Baltimore). 2025 Aug 8;104(32):e43725. doi: 10.1097/MD.0000000000043725. Medicine (Baltimore). 2025. PMID: 40797495 Free PMC article. Review.
-
Associations of sleep disorders with all-cause MCI/dementia and different types of dementia - clinical evidence, potential pathomechanisms and treatment options: A narrative review.Front Neurosci. 2024 Mar 22;18:1372326. doi: 10.3389/fnins.2024.1372326. eCollection 2024. Front Neurosci. 2024. PMID: 38586191 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

