Patient demographics and characteristics from an ambispective, observational study of patients with duchenne muscular dystrophy in Saudi Arabia
- PMID: 36275069
- PMCID: PMC9580328
- DOI: 10.3389/fped.2022.1020059
Patient demographics and characteristics from an ambispective, observational study of patients with duchenne muscular dystrophy in Saudi Arabia
Abstract
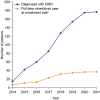
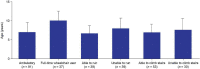
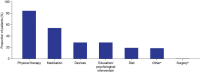
Duchenne muscular dystrophy (DMD) is a rare neuromuscular disorder that is characterized by progressive muscle weakness, resulting in disability and premature death. Onset of symptoms typically occurs at 2-3 years of age, and disease progression is managed through treatment with corticosteroids. The aim of this interim analysis is to increase disease awareness and improve patient management in Saudi Arabia (SA) through the use of data from an ongoing ambispective, observational, multicenter study evaluating characteristics of patients aged 1-14 years with genetically confirmed DMD in SA. This interim analysis examined the secondary outcomes from the study-the demographics and clinical characteristics of patients included retrospectively [data recorded (enrollment visit) between January 2014 and September 2020] and prospectively between September 2020 and April 2021. The primary outcome-the list of DMD gene mutations for the study population-will be reported at a later date. There were 177 eligible patients. Mean, standard deviation (SD) age at enrollment was 7.5 (3.0) years. Median (min, max) age at diagnosis was 7.0 (1.3, 13.8) years. At enrollment, 28.9% of patients were full-time wheelchair users, 50.0% of ambulatory patients could run, and 63.9% could climb stairs. The mean (SD) ages of patients at enrollment who were unable to run and climb stairs were 8.0 (2.7) and 7.6 (3.0) years, respectively. Speech delay (19.4%) and learning difficulties (14.9%) were the most commonly reported intellectual impairments. Physical therapy (84.2%) was the most common choice for initial management of DMD. Only 40.7% of patients received corticosteroid therapy as part of their initial management plan, rising to 59.1% at enrollment. Devices were given to 28.8% of patients for initial management, most commonly ankle-foot orthoses (26.0%) and wheelchairs (6.2%). This analysis reports data from the largest study to date to capture demographics and clinical characteristics of DMD patients in SA. The interim results show a relatively late DMD diagnosis age compared with that in other countries, and a need for improved adherence to international DMD standard of care guidelines. Therefore, there is an urgent requirement for improved DMD education and awareness among healthcare professionals and the public in SA.
Keywords: Saudi Arabia; duchenne muscular dystrophy; dystrophy gene; genetic diagnosis; muscular dystrophy; neuromuscular disorder; patient demographics.
Copyright © 2022 AlSaman, Al Ghamdi, Bamaga, AlShaikh, Al Muqbil, Muthaffar, Bashiri, Ali, Mulayim, Heider, Alshahrani and Al Muhaizea.
Conflict of interest statement
The authors declare that this study received funding from PTC Therapeutics. The funder had the following involvement in the study: contributed to the writing of the article. The concept for manuscript was agreed between the authors and PTC Therapeutics and the decision to submit for publication was made by the authors and PTC Therapeutics. AzA has served as an advisory board member for and received honoraria from Biogen, Genpharm, Novartis, PTC Therapeutics, and Roche. FA has served as an advisory board member for and received honoraria from PTC Therapeutics. NA has served as an advisory board member for and received honoraria from Biogen, Genpharm, Novartis, PTC Therapeutics, Roche, Sanofi Genzyme, and Sarepta Therapeutics and is a member of the National Committee for the Study of Expensive Drugs at the Saudi Health Council. AM and EH were employees of PTC Therapeutics.
Figures

References
-
- Birnkrant DJ, Bushby K, Bann CM, Apkon SD, Blackwell A, Brumbaugh D, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol. (2018) 17:251–67. 10.1016/S1474-4422(18)30024-3 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

