Structure and diversity of mycorrhizal fungi communities of different part of Bulbophyllum tianguii in three terrestrial environments
- PMID: 36275522
- PMCID: PMC9579349
- DOI: 10.3389/fpls.2022.992184
Structure and diversity of mycorrhizal fungi communities of different part of Bulbophyllum tianguii in three terrestrial environments
Abstract
Mycorrhizal fungi plays important roles in the seed germination and subsequent growth of orchids. The research of fungi in orchid roots, especially dominant mycorrhizal fungi is critical for orchids protection. In this study, the fungal community and composition of mycorrhizal fungi in roots, rhizomes and rhizosphere soil of Bulbophyllum tianguii grown in three terrestrial environments were analyzed by the second generation sequencing technology. The results of OTU clustering and α and β diversity analysis showed that there were significant differences in fungal communities in roots, rhizomes and rhizosphere soil of B. tianguii. The total number of OTUs in rhizomes was much less than that in roots and rhizosphere soil. The number of OTUs in rhizosphere soil and the diversity of mycorrhizal fungi were the highest. Meanwhile, the species and abundance of mycorrhizal fungi in roots and rhizomes of B. tianguii were different from those in rhizosphere soil. For different elevations, compared with B. tianguii that grow in middle of Tiankeng and top of Tiankeng, the OTUs number of B. tianguii in orchid garden is richest, and the diversity of mycorrhizal fungi in orchid garden was significantly higher than other locations. Among the three different habitats of B. tianguii, the number of OTUs in humus soil and stone habitats was notably higher than tree habitats, and the diversity of mycorrhizal fungi in humus soil was the highest. The analysis of mycorrhizal fungi in different habitats and altitudes of B. tianguii showed that Sebacina and Exophiala were the dominant mycorrhizal fungi in B. tianguii. The results of species annotation, phylogenetic tree and co-occurrence network analysis showed the dominant mycorrhizal fungi of B. tianguii mainly included Sebacina, Cladosporium, Exophiala, Fusarium. This study reveals the symbiotic relationship between Sebacina, Exophiala, Cladosporium and the B. Tianguii. It will provide a theoretical basis for the protection and biological function study of B. Tianguii.
Keywords: bulbophyllum tianguii; microbial community; mycorrhizal fungi; orchidaceae; orchids.
Copyright © 2022 Liang, Zou, Huang, Qin, Tang, Wei, Liang and Chai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

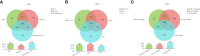

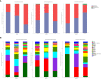

(A-C): root, rhizome and rhizosphere soil groups; (D–F): different habitat groups; (G–I): different altitude groups.



References
-
- Bacaro G., Rocchini D., Ghisla A., Marcantonio M., Neteler M., Chiarucci A. (2012). The spatial domain matters: Spatially constrained species rarefaction in a free and open source environment. Ecol. Complexity. 12, 63–69. doi: 10.1016/j.ecocom.2012.05.007 - DOI
-
- Chen X., Liu Z., Tao G., Zhu Y., Liu Z. (2012). Study on seed germination of Bletilla ochracea improved by mycorrhizal fungi of orchid. Southwest. China J. Agric. Sci. 25 (4), 1393–1397. doi: 10.16213/j.cnki.scjas.2012.04.070 - DOI
-
- Chen Y., Xing X., Guo S. (2017). Nutritional relationships between orchids and mycorrhizal fungi: A review. Mycosystema 36 (7), 807–819. doi: 10.13346/j.mycosystema.160231 - DOI
LinkOut - more resources
Full Text Sources

