H7N9 bearing a mutation in the nucleoprotein leads to increased pathology in chickens
- PMID: 36275684
- PMCID: PMC9583263
- DOI: 10.3389/fimmu.2022.974210
H7N9 bearing a mutation in the nucleoprotein leads to increased pathology in chickens
Abstract
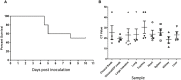
The zoonotic H7N9 avian influenza (AI) virus first emerged in 2013 as a low pathogenic (LPAI) strain, and has repeatedly caused human infection resulting in severe respiratory illness and a mortality of ~39% (>600 deaths) across five epidemic waves. This virus has circulated in poultry with little to no discernible clinical signs, making detection and control difficult. Contrary to published data, our group has observed a subset of specific pathogen free chickens infected with the H7N9 virus succumb to disease, showing clinical signs consistent with highly pathogenic AI (HPAI). Viral genome sequencing revealed two key mutations had occurred following infection in the haemagglutinin (HA 226 L>Q) and nucleoprotein (NP 373 A>T) proteins. We further investigated the impact of the NP mutation and demonstrated that only chickens bearing a single nucleotide polymorphism (SNP) in their IFITM1 gene were susceptible to the H7N9 virus. Susceptible chickens demonstrated a distinct loss of CD8+ T cells from the periphery as well as a dysregulation of IFNγ that was not observed for resistant chickens, suggesting a role for the NP mutation in altered T cell activation. Alternatively, it is possible that this mutation led to altered polymerase activity, as the mutation occurs in the NP 360-373 loop which has been previously show to be important in RNA binding. These data have broad ramifications for our understanding of the pathobiology of AI in chickens and humans and provide an excellent model for investigating the role of antiviral genes in a natural host species.
Keywords: H7N9; Mutation; T cell; influenza; interferon.
Copyright © 2022 Layton, Butler, Stewart, Stevens, Payne, Rootes, Deffrasnes, Walker, Shan, Gough, Cowled, Bruce, Wang, Kedzierska, Wong, Bean, Bingham and Williams.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

