Lipids for CD8+ TILs: Beneficial or harmful?
- PMID: 36275711
- PMCID: PMC9585227
- DOI: 10.3389/fimmu.2022.1020422
Lipids for CD8+ TILs: Beneficial or harmful?
Abstract
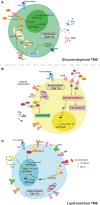
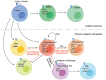
Lipids and lipid metabolism play crucial roles in regulating T cell function and are tightly related to the establishment of immune memory. It is reported that tumor-infiltrating CD8+T lymphocytes (CD8+TILs) burn fats to restore their impaired effector function due to the lack of glucose. Conversely, fatty acids (FAs) and cholesterol in the tumor microenvironment (TME) drive the CD8+ TILs dysfunction. The origin of dysfunctional CD8+ TILs shares important features with memory T cell's precursor, but whether lipids and/or lipid metabolism reprogramming directly influence the memory plasticity of dysfunctional CD8+ TILs remains elusive. It is necessary to understand the interplay between cellular lipid metabolism and dysfunction of CD8+ TILs in the case of targeting T cell's metabolism to synergize cancer immunotherapy. Therefore, in this review, we summarize the latest research on CD8+ TILs lipid metabolism, evaluate the impacts of lipids in the TME to CD8+ TILs, and highlight the significance of promoting memory phenotype cell formation by targeting CD8+ T cells lipid metabolism to provide longer duration of cancer immunotherapy efficacy.
Keywords: CD8+ T cell; Exhausted T cell; cholesterol; fatty acid; fatty acid oxidation; metabolism; tumor microenvironment (TME).
Copyright © 2022 Wu and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Enhancing CD8+ T Cell Fatty Acid Catabolism within a Metabolically Challenging Tumor Microenvironment Increases the Efficacy of Melanoma Immunotherapy.Cancer Cell. 2017 Sep 11;32(3):377-391.e9. doi: 10.1016/j.ccell.2017.08.004. Cancer Cell. 2017. PMID: 28898698 Free PMC article.
-
Uptake of oxidized lipids by the scavenger receptor CD36 promotes lipid peroxidation and dysfunction in CD8+ T cells in tumors.Immunity. 2021 Jul 13;54(7):1561-1577.e7. doi: 10.1016/j.immuni.2021.05.003. Epub 2021 Jun 7. Immunity. 2021. PMID: 34102100 Free PMC article.
-
CD226 identifies functional CD8+T cells in the tumor microenvironment and predicts a better outcome for human gastric cancer.Front Immunol. 2023 Mar 28;14:1150803. doi: 10.3389/fimmu.2023.1150803. eCollection 2023. Front Immunol. 2023. PMID: 37056782 Free PMC article.
-
Metabolic immunoengineering approaches to enhance CD8+ T cell-based cancer immunotherapy.Cell Syst. 2024 Dec 18;15(12):1225-1244. doi: 10.1016/j.cels.2024.11.010. Cell Syst. 2024. PMID: 39701038 Review.
-
The role of tumor-infiltrating lymphocytes in cholangiocarcinoma.J Exp Clin Cancer Res. 2022 Apr 7;41(1):127. doi: 10.1186/s13046-022-02340-2. J Exp Clin Cancer Res. 2022. PMID: 35392957 Free PMC article. Review.
Cited by
-
SCD1 inhibition enhances the effector functions of CD8+ T cells via ACAT1-dependent reduction of esterified cholesterol.Cancer Sci. 2024 Jan;115(1):48-58. doi: 10.1111/cas.15999. Epub 2023 Oct 25. Cancer Sci. 2024. PMID: 37879607 Free PMC article.
-
Abnormal changes in metabolites caused by m6A methylation modification: The leading factors that induce the formation of immunosuppressive tumor microenvironment and their promising potential for clinical application.J Adv Res. 2025 Apr;70:159-186. doi: 10.1016/j.jare.2024.04.016. Epub 2024 Apr 25. J Adv Res. 2025. PMID: 38677545 Free PMC article. Review.
-
Cholesterol metabolism reprogramming in multiple myeloma: examining its specificity and impact on the immune microenvironment.Am J Cancer Res. 2025 May 15;15(5):2004-2021. doi: 10.62347/CCCT1933. eCollection 2025. Am J Cancer Res. 2025. PMID: 40520860 Free PMC article. Review.
-
Developing and validating the model of tumor-infiltrating immune cell to predict survival in patients receiving radiation therapy for head and neck squamous cell carcinoma.Transl Cancer Res. 2024 Jan 31;13(1):394-412. doi: 10.21037/tcr-23-2345. Epub 2024 Jan 29. Transl Cancer Res. 2024. PMID: 38410204 Free PMC article.
-
New insights into T cell metabolism in liver cancer: from mechanism to therapy.Cell Death Discov. 2025 Mar 23;11(1):118. doi: 10.1038/s41420-025-02397-w. Cell Death Discov. 2025. PMID: 40122853 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

