Selective serotonin reuptake inhibitors and inflammatory bowel disease; Beneficial or malpractice
- PMID: 36275739
- PMCID: PMC9583131
- DOI: 10.3389/fimmu.2022.980189
Selective serotonin reuptake inhibitors and inflammatory bowel disease; Beneficial or malpractice
Abstract
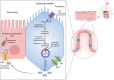
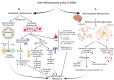
IBD, a chronic inflammatory disease, has been manifested as a growing health problem. No Crohn's and Colitis councils have officially ratified anti-depressants as a routine regimen for IBD patients. However, some physicians empirically prescribe them to rectify functional bowel consequences such as pain and alleviate psychiatric comorbidities. On the other side, SSRIs' prescription is accompanied by adverse effects such as sleep disturbances. Prolonged intermittent hypoxia throughout sleep disturbance such as sleep apnea provokes periodic reductions in the partial oxygen pressure gradient in the gut lumen. It promotes gut microbiota to dysbiosis, which induces intestinal inflammation. This phenomenon and evidence representing the higher amount of serotonin associated with Crohn's disease challenged our previous knowledge. Can SSRIs worsen the IBD course? Evidence answered the question with the claim on anti-inflammatory properties (central and peripheral) of SSRIs and illuminated the other substantial elements (compared to serotonin elevation) responsible for IBD pathogenesis. However, later clinical evidence was not all in favor of the benefits of SSRIs. Hence, in this review, the molecular mechanisms and clinical evidence are scrutinized and integrated to clarify the interfering molecular mechanism justifying both supporting and disproving clinical evidence. Biphasic dose-dependent serotonin behavior accompanying SSRI shifting function when used up for the long-term can be assumed as the parameters leading to IBD patients' adverse outcomes. Despite more research being needed to elucidate the effect of SSRI consumption in IBD patients, periodic prescriptions of SSRIs at monthly intervals can be recommended.
Keywords: Crohn’s disease; anti-inflammatory; antidepressant; dysbiosis; inflammatory bowel diseases; pro-inflammatory; serotonin uptake inhibitors; ulcerative colitis.
Copyright © 2022 Hatamnejad, Baradaran Ghavami, Shirvani, Asghari Ahmadabad, Shahrokh, Farmani, Sherkat, Asadzadeh Aghdaei and Zali.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Mikocka-Walus AA, Turnbull DA, Andrews JM, Moulding NT, Holtmann GJ. The effect of functional gastrointestinal disorders on psychological comorbidity and quality of life in patients with inflammatory bowel disease. Aliment Pharmacol Ther (2008) 28(4):475–83. doi: 10.1111/j.1365-2036.2008.03754.x - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

