IL-17 and IL-22 are pivotal cytokines to delay wound healing of S. aureus and P. aeruginosa infected skin
- PMID: 36275755
- PMCID: PMC9585169
- DOI: 10.3389/fimmu.2022.984016
IL-17 and IL-22 are pivotal cytokines to delay wound healing of S. aureus and P. aeruginosa infected skin
Abstract
Introduction: Although the presence of pathogens in skin wounds is known to delay the wound healing process, the mechanisms underlying this delay remain poorly understood. In the present study, we have investigated the regulatory role of proinflammatory cytokines on the healing kinetics of infected wounds.
Methods: We have developed a mouse model of cutaneous wound healing, with or without wound inoculation with Staphylococcus aureus and Pseudomonas aeruginosa, two major pathogens involved in cutaneous wound bacterial infections.
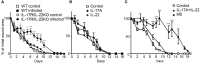
Results: Aseptic excision in C57BL/6 mouse skin induced early expression of IL-1β, TNFα and Oncostatin M (OSM), without detectable expression of IL-22 and IL-17A/F. S. aureus and P. aeruginosa wound inoculation not only increased the expression of IL-1β and OSM, but also induced a strong cutaneous expression of IL-22, IL-17A and IL-17F, along with an increased number of infiltrating IL-17A and/or IL-22-producing γδ T cells. The same cytokine expression pattern was observed in infected human skin wounds. When compared to uninfected wounds, mouse skin infection delayed the wound healing process. Injection of IL-1α, TNFα, OSM, IL-22 and IL-17 together in the wound edges induced delayed wound healing similar to that induced by the bacterial infection. Wound healing experiments in infected Rag2KO mice (deficient in lymphocytes) showed a wound healing kinetic similar to uninfected Rag2KO mice or WT mice. Rag2KO infected-skin lesions expressed lower levels of IL-17 and IL-22 than WT, suggesting that the expression of these cytokines is mainly dependent on γδ T cells in this model. Wound healing was not delayed in infected IL-17R/IL-22KO, comparable to uninfected control mice. Injection of recombinant IL-22 and IL-17 in infected wound edges of Rag2KO mice re-establish the delayed kinetic of wound healing, as in infected WT mice.
Conclusion: These results demonstrate the synergistic and specific effects of IL-22 and IL-17 induced by bacterial infection delay the wound healing process, regardless of the presence of bacteria per se. Therefore, these cytokines play an unexpected role in delayed skin wound healing.
Keywords: Th17; cytokine; inflammation; pathogen; skin; wound healing.
Copyright © 2022 Lecron, Charreau, Jégou, Salhi, Petit-Paris, Guignouard, Burucoa, Favot-Laforge, Bodet, Barra, Huguier, Mcheik, Dumoutier, Garnier, Bernard, Ryffel and Morel.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

