Amino acid metabolism in primary bone sarcomas
- PMID: 36276057
- PMCID: PMC9581121
- DOI: 10.3389/fonc.2022.1001318
Amino acid metabolism in primary bone sarcomas
Abstract
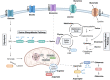
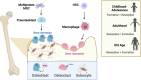
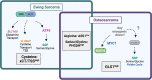
Primary bone sarcomas, including osteosarcoma (OS) and Ewing sarcoma (ES), are aggressive tumors with peak incidence in childhood and adolescence. The intense standard treatment for these patients consists of combined surgery and/or radiation and maximal doses of chemotherapy; a regimen that has not seen improvement in decades. Like other tumor types, ES and OS are characterized by dysregulated cellular metabolism and a rewiring of metabolic pathways to support the biosynthetic demands of malignant growth. Not only are cancer cells characterized by Warburg metabolism, or aerobic glycolysis, but emerging work has revealed a dependence on amino acid metabolism. Aside from incorporation into proteins, amino acids serve critical functions in redox balance, energy homeostasis, and epigenetic maintenance. In this review, we summarize current studies describing the amino acid metabolic requirements of primary bone sarcomas, focusing on OS and ES, and compare these dependencies in the normal bone and malignant tumor contexts. We also examine insights that can be gleaned from other cancers to better understand differential metabolic susceptibilities between primary and metastatic tumor microenvironments. Lastly, we discuss potential metabolic vulnerabilities that may be exploited therapeutically and provide better-targeted treatments to improve the current standard of care.
Keywords: Ewing sarcoma; amino acid metabolism; osteoblast; osteoclast; sarcoma; tumor metabolism.
Copyright © 2022 Jiménez, Lawlor and Lyssiotis.
Conflict of interest statement
CL has received consulting fees from Astellas Pharmaceuticals, Odyssey Therapeutics, and T-Knife Therapeutics, and is an inventor on patents pertaining to Kras regulated metabolic pathways, redox control pathways in pancreatic cancer, and targeting the GOT1-pathway as a therapeutic approach (US Patent No: 2015126580-A1, 05/07/2015; US Patent No: 20190136238, 05/09/2019; International Patent No: WO2013177426-A2, 04/23/2015). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

