DNAAF5 promotes hepatocellular carcinoma malignant progression by recruiting USP39 to improve PFKL protein stability
- PMID: 36276075
- PMCID: PMC9582515
- DOI: 10.3389/fonc.2022.1032579
DNAAF5 promotes hepatocellular carcinoma malignant progression by recruiting USP39 to improve PFKL protein stability
Abstract
Purposes: Dynein axonemal assembly factor 5 (DNAAF5) is the transcription factor of regulating the cytoskeleton and hydrodynamic protein complex assembly, however, it was not well elucidated in the malignant progression of hepatocellular carcinoma (HCC).
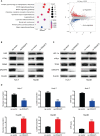
Methods: We investigated the role of DNAAF5 in hepatocellular carcinoma by using multiple groups of clinical tissues combined with data from the TCGA database. Then we overexpressed DNAAF5 in hepatocellular carcinoma tumor tissues, which correlates with poor patient survival outcomes. Furthermore, we constructed stable cell lines of HCC cells to confirm the cancer-promoting effects of DNAAF5 in hepatocellular carcinoma. To explore the mechanisms of DNAAF5, transcriptome sequencing combined with mass spectrometry was also performed, which showed that DNAAF5 affects its downstream signaling pathway by interacting with PFKL and that DNAAF5 regulates PFKL protein stability by recruiting the deubiquitination protein, USP39. To corroborate these findings, the same series of tissue microarrays were used to confirm correlations between DNAAF5 and PFKL expressions. In animal experiments, DNAAF5 also promoted the proliferation of HCC cells.
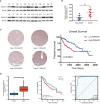
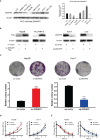
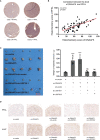
Results: We found that DNAAF5 expressions were markedly higher in HCC tissues, compared to the adjacent normal tissues. Increased levels of DNAAF5 were associated with significantly worse prognostic outcomes for HCC patients. Cell function experiments showed that HCC cells of overexpressing DNAAF5 exhibited faster proliferation rates, stronger clone formation abilities and higher drug resistance rates. However, tumor cell proliferation rates and colony formation were significantly decreased after DNAAF5 knockout, accompanied by an increase in sensitivity to sorafenib. In addition, the results of our study showed that DNAAF5 accelerates PFKL protein deubiquitination by recruiting USP39 in HCC cells. Furthermore, The overexpression of DNAAF5 could promote HCC cell proliferation in vivo and in vitro, whereas USP39 knockdown inhibited this effect. Overall, DNAAF5 serves as a scaffold protein to recruit USP39 to form a ternary complex by directly binding the PFKL protein, thereby improving the stability of the latter, which promotes the malignant process of hepatocellular carcinoma.
Conclusions: These findings revealed DNAAF5 was negatively correlated with the prognosis of patients with hepatocellular carcinoma. It underlying mechanism showed that DNAAF5 directly binds PFKL and recruits the deubiquitinated protein (USP39) to improve the stability of the PFKL protein, thus enhancing abnormal glycolysis in HCC cells.
Keywords: DNAAF5; HCC; PFKL protein; glycolysis; prognosis.
Copyright © 2022 Liu, Wu, Sun, Huang, Han and Han.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Exploring the cancerous nexus: the pivotal and diverse roles of USP39 in cancer development.Discov Oncol. 2025 May 10;16(1):715. doi: 10.1007/s12672-025-02480-9. Discov Oncol. 2025. PMID: 40347416 Free PMC article. Review.
-
A functional loop between YTH domain family protein YTHDF3 mediated m6A modification and phosphofructokinase PFKL in glycolysis of hepatocellular carcinoma.J Exp Clin Cancer Res. 2022 Dec 6;41(1):334. doi: 10.1186/s13046-022-02538-4. J Exp Clin Cancer Res. 2022. PMID: 36471428 Free PMC article.
-
A20 targets PFKL and glycolysis to inhibit the progression of hepatocellular carcinoma.Cell Death Dis. 2020 Feb 3;11(2):89. doi: 10.1038/s41419-020-2278-6. Cell Death Dis. 2020. PMID: 32015333 Free PMC article.
-
USP39 promotes the growth of human hepatocellular carcinoma in vitro and in vivo.Oncol Rep. 2015 Aug;34(2):823-32. doi: 10.3892/or.2015.4065. Epub 2015 Jun 15. Oncol Rep. 2015. PMID: 26081192
-
USP39 promote post-translational modifiers to stimulate the progress of cancer.Discov Oncol. 2025 May 13;16(1):749. doi: 10.1007/s12672-025-02573-5. Discov Oncol. 2025. PMID: 40358671 Free PMC article. Review.
Cited by
-
RBM12 drives PD-L1-mediated immune evasion in hepatocellular carcinoma by increasing JAK1 mRNA translation.Oncogene. 2024 Oct;43(41):3062-3077. doi: 10.1038/s41388-024-03140-y. Epub 2024 Aug 26. Oncogene. 2024. PMID: 39187545
-
Toosendanin promotes prostate cancer cell apoptosis, ferroptosis and M1 polarization via USP39-mediated PLK1 deubiquitination.Naunyn Schmiedebergs Arch Pharmacol. 2025 Aug;398(8):10939-10949. doi: 10.1007/s00210-025-03916-3. Epub 2025 Mar 8. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 40056202
-
USP39: a key regulator in malignant tumor progression.Front Oncol. 2025 Jul 2;15:1556011. doi: 10.3389/fonc.2025.1556011. eCollection 2025. Front Oncol. 2025. PMID: 40672756 Free PMC article. Review.
-
Exploring the cancerous nexus: the pivotal and diverse roles of USP39 in cancer development.Discov Oncol. 2025 May 10;16(1):715. doi: 10.1007/s12672-025-02480-9. Discov Oncol. 2025. PMID: 40347416 Free PMC article. Review.
-
EGR1 suppresses HCC growth and aerobic glycolysis by transcriptionally downregulating PFKL.J Exp Clin Cancer Res. 2024 Jan 29;43(1):35. doi: 10.1186/s13046-024-02957-5. J Exp Clin Cancer Res. 2024. PMID: 38287371 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

