Hepatic macrophage mediated immune response in liver steatosis driven carcinogenesis
- PMID: 36276076
- PMCID: PMC9581256
- DOI: 10.3389/fonc.2022.958696
Hepatic macrophage mediated immune response in liver steatosis driven carcinogenesis
Abstract
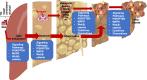
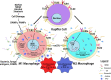
Obesity confers an independent risk for carcinogenesis. Classically viewed as a genetic disease, owing to the discovery of tumor suppressors and oncogenes, genetic events alone are not sufficient to explain the progression and development of cancers. Tumor development is often associated with metabolic and immunological changes. In particular, obesity is found to significantly increase the mortality rate of liver cancer. As its role is not defined, a fundamental question is whether and how metabolic changes drive the development of cancer. In this review, we will dissect the current literature demonstrating that liver lipid dysfunction is a critical component driving the progression of cancer. We will discuss the involvement of inflammation in lipid dysfunction driven liver cancer development with a focus on the involvement of liver macrophages. We will first discuss the association of steatosis with liver cancer. This will be followed with a literature summary demonstrating the importance of inflammation and particularly macrophages in the progression of liver steatosis and highlighting the evidence that macrophages and macrophage produced inflammatory mediators are critical for liver cancer development. We will then discuss the specific inflammatory mediators and their roles in steatosis driven liver cancer development. Finally, we will summarize the molecular pattern (PAMP and DAMP) as well as lipid particle signals that are involved in the activation, infiltration and reprogramming of liver macrophages. We will also discuss some of the therapies that may interfere with lipid metabolism and also affect liver cancer development.
Keywords: Kupffer Cells; inflammation; liver cancer; macrophages; steatosis.
Copyright © 2022 Tu, Alba, Datta, Hong, Hua, Jia, Khan, Nguyen, Niu, Pammidimukkala, Slarve, Tang, Xu, Zhou and Stiles.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Vitamin D Receptor Activation in Liver Macrophages Ameliorates Hepatic Inflammation, Steatosis, and Insulin Resistance in Mice.Hepatology. 2020 May;71(5):1559-1574. doi: 10.1002/hep.30937. Epub 2020 Feb 23. Hepatology. 2020. PMID: 31506976
-
Wnt/β-catenin activation and macrophage induction during liver cancer development following steatosis.Oncogene. 2017 Oct 26;36(43):6020-6029. doi: 10.1038/onc.2017.207. Epub 2017 Jul 3. Oncogene. 2017. PMID: 28671671 Free PMC article.
-
Cooperation of liver cells in health and disease.Adv Anat Embryol Cell Biol. 2001;161:III-XIII, 1-151. doi: 10.1007/978-3-642-56553-3. Adv Anat Embryol Cell Biol. 2001. PMID: 11729749 Review.
-
Macrophages: The Good, the Bad, and the Gluttony.Front Immunol. 2021 Aug 12;12:708186. doi: 10.3389/fimmu.2021.708186. eCollection 2021. Front Immunol. 2021. PMID: 34456917 Free PMC article. Review.
-
Macrophages in obesity and non-alcoholic fatty liver disease: Crosstalk with metabolism.JHEP Rep. 2019 Feb 23;1(1):30-43. doi: 10.1016/j.jhepr.2019.02.004. eCollection 2019 May. JHEP Rep. 2019. PMID: 32149275 Free PMC article. Review.
Cited by
-
Diverse Functions of Macrophages in Obesity and Metabolic Dysfunction-Associated Steatotic Liver Disease: Bridging Inflammation and Metabolism.Immune Netw. 2025 Feb 21;25(1):e12. doi: 10.4110/in.2025.25.e12. eCollection 2025 Feb. Immune Netw. 2025. PMID: 40078789 Free PMC article. Review.
-
Eicosanoids and other oxylipins in liver injury, inflammation and liver cancer development.Front Physiol. 2023 Feb 2;14:1098467. doi: 10.3389/fphys.2023.1098467. eCollection 2023. Front Physiol. 2023. PMID: 36818443 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

