Impact of cryopreservation on CAR T production and clinical response
- PMID: 36276077
- PMCID: PMC9582437
- DOI: 10.3389/fonc.2022.1024362
Impact of cryopreservation on CAR T production and clinical response
Abstract
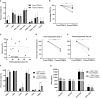
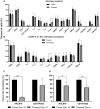
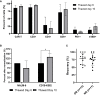
Adoptive cell therapy with chimeric antigen receptor (CAR) T cells has become an efficient treatment option for patients with hematological malignancies. FDA approved CAR T products are manufactured in centralized facilities from fresh or frozen leukapheresis and the cryopreserved CAR T infusion product is shipped back to the patient. An increasing number of clinical centers produce CAR T cells on-site, which enables the use of fresh and cryopreserved PBMCs and CAR T cells. Here we determined the effect of cryopreservation on PBMCs and CD19 CAR T cells in a cohort of 118 patients treated with fresh CAR T cells and in several patients head-to-head. Cryopreserved PBMCs, obtained from leukapheresis products, contained less erythrocytes and T cells, but were sufficient to produce CAR T cells for therapy. There was no correlation between the recovery of PBMCs and the transduction efficacy, the number of CAR T cells obtained by the end of the manufacturing process, the in vitro reactivity, or the response rate to CAR T therapy. We could show that CAR T cells cryopreserved during the manufacturing process, stored and resumed expansion at a later time point, yielded sufficient cell numbers for treatment and led to complete remissions. Phenotype analysis including T cell subtypes, chemokine receptor and co-inhibitory/stimulatory molecules, revealed that fresh CAR T cells expressed significantly more TIM-3 and contained less effector T cells in comparison to their frozen counterparts. In addition, fresh CAR T infusion products demonstrated increased in vitro anti-tumor reactivity, however cryopreserved CAR T cells still showed high anti-tumor potency and specificity. The recovery of cryopreserved CAR T cells was similar in responding and non-responding patients. Although fresh CAR T infusion products exhibit higher anti-tumor reactivity, the use of frozen PBMCs as staring material and frozen CAR T infusion products seems a viable option, as frozen products still exhibit high in vitro potency and cryopreservation did not seem to affect the clinical outcome.
Keywords: CAR T cells; CAR T manufacturing; PBMC; clinical trial; cryopreservation.
Copyright © 2022 Brezinger-Dayan, Itzhaki, Melnichenko, Kubi, Zeltzer, Jacoby, Avigdor, Shapira Frommer and Besser.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials

