Inhibition of induced-hepatic cancer in vivo through IQGAP1-shRNA gene therapy and modulation of TRAIL-induced apoptosis pathway
- PMID: 36276098
- PMCID: PMC9581201
- DOI: 10.3389/fonc.2022.998247
Inhibition of induced-hepatic cancer in vivo through IQGAP1-shRNA gene therapy and modulation of TRAIL-induced apoptosis pathway
Abstract
Background: Liver cancer is the deadliest malignancy among common tumors. It is the top cause of cancer-related deaths in Egypt, and it is characterized by increasing occurrence among the population. The objective of this study was to determine the outcome of pre-treatment of IQGAP1-shRNA on induced mouse hepatocellular carcinoma model and evaluate the potency of this IQGAP1-shRNA plasmid to recover hepatic cancer as a new tool of cancer therapy. Therefore, we will use RNA interference (RNAi) technology to silence IQGAP1 oncogene to completely recover the chemically induced models for hepatic cancer by designing short RNAi specific for IQGAP1 gene in HCC cells in vivo and construct new vectors suitable for this purpose. We assigned mice into three groups: the first negative control group (NC) was injected with saline, the second control group was injected with shRNA (shNC), the third positive control group was injected with diethylnitrosamine (DENAA), and the fourth group was treated with the IQGAP1-shRNA prior to its exposure to DENA.
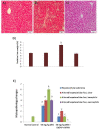
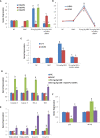
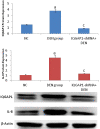
Results: Our results revealed that the treated group with IQGAP1-shRNA with DENA developed very few cases of hepatic cancer when compared with the positive control group. The positive control group exhibited significant increases in the liver function level as well as a decrease in serum albumin levels when compared to both the treated and the negative control groups. The altered levels of the serum α-fetoprotein as well as of the tumor necrosis factor-alpha, and interleukin-4 in DENA-treated mice were significantly ameliorated by IQGAP1-shRNA administration. Flow cytometer analyses have indicated that the silencing of IQGAP1 cannot significantly modulate DENA-induced apoptosis in the circulating blood cells. Moreover, the elevated mRNA expression levels of IQGAP1, IQGAP3, KRas, HRas, interleukin-8, nuclear factor kappa B, caspase-3, caspase-9 and Bcl-2, were significantly decreased by the IQGAP1-shRNA treatment. However, the IQGAP2, DR4, DR5, p53 and BAX genes were found to be significantly up-regulated post-therapy. In agreement with these findings, IQGAP1-shRNA was able to modulate the DENA-induced histological changes in the mice liver which were represented by severe necrosis and hydropic degenerative changes.
Conclusion: Our study revealed that IQGAP1-shRNA was able to preserve hepatocyte integrity and the liver histological architecture through the regulation of the expression of IQGAPs, Ras, TRAILs and IL-8 receptors, as well as of pro-apoptotic and anti-apoptotic genes. Therefore, the silencing of IQGAP1 could be part of a promising therapeutic strategy against hepatic cancer.
Keywords: IQGAP1; in vivo; liver cancer; mouse; shRNA.
Copyright © 2022 Zoheir, Abd-Rabou, Darwish, Abdelhafez and Mahrous.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Novel Approach Using shRNA of IQGAP1 for Colon Cancer Therapy: HCT116 as a Surrogate Model Colorectal Carcinoma.Asian Pac J Cancer Prev. 2022 Jul 1;23(7):2387-2395. doi: 10.31557/APJCP.2022.23.7.2387. Asian Pac J Cancer Prev. 2022. PMID: 35901346 Free PMC article.
-
IQGAP1 gene silencing induces apoptosis and decreases the invasive capacity of human hepatocellular carcinoma cells.Tumour Biol. 2016 Oct;37(10):13927-13939. doi: 10.1007/s13277-016-5283-8. Epub 2016 Aug 3. Tumour Biol. 2016. PMID: 27488117
-
New Approach about the Signaling Crosstalk between IQGAPs/ NF- κB/IL-8 and PDCD5/p53/TRAIL Pathways that Modulate Malignant Transformation in Hepatocellular Carcinoma.Asian Pac J Cancer Prev. 2022 Jan 1;23(1):271-279. doi: 10.31557/APJCP.2022.23.1.271. Asian Pac J Cancer Prev. 2022. PMID: 35092397 Free PMC article.
-
The Antithetic Roles of IQGAP2 and IQGAP3 in Cancers.Cancers (Basel). 2023 Feb 9;15(4):1115. doi: 10.3390/cancers15041115. Cancers (Basel). 2023. PMID: 36831467 Free PMC article. Review.
-
Role of IQ Motif-Containing GTPase-Activating Proteins in Hepatocellular Carcinoma.Front Oncol. 2022 Jun 16;12:920652. doi: 10.3389/fonc.2022.920652. eCollection 2022. Front Oncol. 2022. PMID: 35785216 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

