Differentiating cellular leiomyoma from uterine sarcoma and atypical leiomyoma using multi-parametric MRI
- PMID: 36276145
- PMCID: PMC9582758
- DOI: 10.3389/fonc.2022.1005191
Differentiating cellular leiomyoma from uterine sarcoma and atypical leiomyoma using multi-parametric MRI
Abstract
Objectives: To evaluate the diagnostic performance of conventional magnetic resonance imaging (cMRI) combined with diffusion-weighted MRI (DWI) in discrimination of cellular leiomyoma, uterine sarcoma, and atypical leiomyoma.
Methods: This retrospective study enrolled 106 patients with uterine masses, including 51 cellular leiomyomas (CLs), 32 uterine sarcomas (USs) and 23 degenerated leiomyomas (LMs) confirmed by histopathologic examination. Clinical data and imaging findings were assessed. Chi-squared test for qualitative variables and one way ANOVA analysis for quantitative variables were performed. Logistic regression analysis and the receiver operating characteristic (ROC) analysis were performed to determine the cut-off point and diagnostic performances for significant numeric values or multiple models.
Results: Morphology (Odds ratio [OR] = 6.36) and margin (OR = 13.84) derived from cMRI were independent indicators for differentiating CLs from USs, and T2WI signal (OR = 0.23) were an independent indicator for differentiating CLs from degenerated LMs (all P < 0.05). The cutoff value of apparent diffusion coefficient (ADC) derived from DWI for differentiating CLs from USs was 839 ×10-6 mm2/sec and was 1239 ×10-6 mm2/sec for differentiating CLs from degenerated LMs. Compared with the use of cMRI features and ADC value alone, combination of independent indicators and ADC value achieved higher AUCs for both differentiations (all P < 0.05).
Conclusions: cMRI is a reliable tool for differentiating CLs from USs and atypical leiomyoma, especially degenerated LMs. The combined use of cMRI and DWI can improve the differential diagnostic performance.
Keywords: atypical leiomyoma; diffusion-weighted MRI; magnetic resonance imaging; uterine leiomyoma; uterine sarcoma.
Copyright © 2022 Wang, Zheng, Zhou, Shi, Wu and Lin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

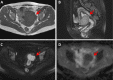

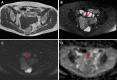

Similar articles
-
Differentiating Uterine Sarcoma From Atypical Leiomyoma on Preoperative Magnetic Resonance Imaging Using Logistic Regression Classifier: Added Value of Diffusion-Weighted Imaging-Based Quantitative Parameters.Korean J Radiol. 2024 Jan;25(1):43-54. doi: 10.3348/kjr.2023.0760. Korean J Radiol. 2024. PMID: 38184768 Free PMC article.
-
Preoperative diagnosis of atypical pelvic leiomyoma and sarcoma: the potential role of diffusion-weighted imaging.J Obstet Gynaecol. 2019 Jan;39(1):98-104. doi: 10.1080/01443615.2018.1466110. Epub 2018 Sep 12. J Obstet Gynaecol. 2019. PMID: 30207503
-
Diagnostic Algorithm to Differentiate Benign Atypical Leiomyomas from Malignant Uterine Sarcomas with Diffusion-weighted MRI.Radiology. 2020 Nov;297(2):361-371. doi: 10.1148/radiol.2020191658. Epub 2020 Sep 15. Radiology. 2020. PMID: 32930650
-
Utility of ADC Values for Differentiating Uterine Sarcomas From Leiomyomas: Systematic Review and Meta-Analysis.AJR Am J Roentgenol. 2024 Sep;223(3):e2431280. doi: 10.2214/AJR.24.31280. Epub 2024 Jun 5. AJR Am J Roentgenol. 2024. PMID: 38899844
-
Differentiating uterine sarcoma from leiomyoma: BET1T2ER Check!Br J Radiol. 2021 Sep 1;94(1125):20201332. doi: 10.1259/bjr.20201332. Epub 2021 May 5. Br J Radiol. 2021. PMID: 33684303 Free PMC article. Review.
Cited by
-
The utility of MRI for the preoperative differential diagnosis of uterine sarcoma and leiomyoma: a single-center study.Fukushima J Med Sci. 2024 Oct 18;70(4):211-218. doi: 10.5387/fms.23-00018. Epub 2024 Oct 4. Fukushima J Med Sci. 2024. PMID: 39370272 Free PMC article.
-
Recurrence complicated with peritoneal dissemination after single-port gasless myomectomy for cellular uterine leiomyoma: A case report and literature review.Medicine (Baltimore). 2024 Mar 15;103(11):e37444. doi: 10.1097/MD.0000000000037444. Medicine (Baltimore). 2024. PMID: 38489723 Free PMC article. Review.
-
Clinical Features and Imaging Findings of Low-Grade Endometrial Stromal Sarcoma: A Retrospective Case Series-Based Analysis.Cureus. 2025 Mar 13;17(3):e80507. doi: 10.7759/cureus.80507. eCollection 2025 Mar. Cureus. 2025. PMID: 40225539 Free PMC article.
-
Abnormal Uterine Bleeding: A Pictorial Review on Differential Diagnosis and Not-So-Common Cases of Interventional Radiology Management.Diagnostics (Basel). 2024 Apr 11;14(8):798. doi: 10.3390/diagnostics14080798. Diagnostics (Basel). 2024. PMID: 38667444 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

