CUB Domain-Containing Protein 1 (CDCP1) is a rational target for the development of imaging tracers and antibody-drug conjugates for cancer detection and therapy
- PMID: 36276654
- PMCID: PMC9576610
- DOI: 10.7150/thno.78171
CUB Domain-Containing Protein 1 (CDCP1) is a rational target for the development of imaging tracers and antibody-drug conjugates for cancer detection and therapy
Abstract
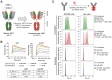
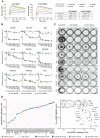
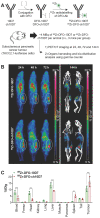
Rationale: An antibody-drug conjugate (ADC) is a targeted therapy consisting of a cytotoxic payload that is linked to an antibody which targets a protein enriched on malignant cells. Multiple ADCs are currently used clinically as anti-cancer agents significantly improving patient survival. Herein, we evaluated the rationale of targeting the cell surface oncoreceptor CUB domain-containing protein 1 (CDCP1) using ADCs and assessed the efficacy of CDCP1-directed ADCs against a range of malignant tumors. Methods: CDCP1 mRNA expression was evaluated using large transcriptomic datasets of normal/tumor samples for 23 types of cancer and 15 other normal organs, and CDCP1 protein expression was examined in 34 normal tissues, >300 samples from six types of cancer, and in 49 cancer cell lines. A recombinant human/mouse chimeric anti-CDCP1 antibody (ch10D7) was labelled with 89Zirconium or monomethyl auristatin E (MMAE) and tested in multiple pre-clinical cancer models including 36 cancer cell lines and three mouse xenograft models. Results: Analysis of CDCP1 expression indicates elevated CDCP1 expression in the majority of the cancers and restricted expression in normal human tissues. Antibody ch10D7 demonstrates a high affinity and specificity for CDCP1 inducing cell signalling via Src accompanied by rapid internalization of ch10D7/CDCP1 complexes in cancer cells. 89Zirconium-labelled ch10D7 accumulates in CDCP1 expressing cells enabling detection of pancreatic cancer xenografts in mice by PET imaging. Cytotoxicity of MMAE-labelled ch10D7 against kidney, colorectal, lung, ovarian, pancreatic and prostate cancer cells in vitro, correlates with the level of CDCP1 on the plasma membrane. ch10D7-MMAE displays robust anti-tumor effects against mouse xenograft models of pancreatic, colorectal and ovarian cancer. Conclusion: CDCP1 directed imaging agents will be useful for selecting cancer patients for personalized treatment with cytotoxin-loaded CDCP1 targeting agents including antibody-drug conjugates.
Keywords: CDCP1; antibody-drug conjugate; cancer; receptor; theranostics.
© The author(s).
Conflict of interest statement
Competing Interests: Thomas Kryza, Simon Puttick, Yaowu He and John D. Hooper are inventors on a patent covering the use of antibody 10D7 used in this study. No potential conflicts of interest were disclosed by the other authors.
Figures



References
-
- Arnold C. Theranostics could be big business in precision oncology. Nat Med. 2022;28:606–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

