The impact of medication on osseointegration and implant anchorage in bone determined using removal torque-A review
- PMID: 36276721
- PMCID: PMC9582727
- DOI: 10.1016/j.heliyon.2022.e10844
The impact of medication on osseointegration and implant anchorage in bone determined using removal torque-A review
Abstract
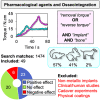
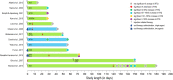
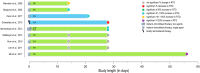
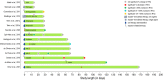
Permanently anchored metal implants are frequently used in dental, craniomaxillofacial, and orthopaedic rehabilitation. The success of such therapies is owed to the phenomenon of osseointegration-the direct connection between the living bone and the implant. The extent of biomechanical anchorage (i.e., physical interlocking between the implant and bone) can be assessed with removal torque (RTQ) measurement. Implant anchorage is strongly influenced by underlying bone quality, involving physicochemical and biological properties such as composition and structural organisation of extracellular matrix, extent of micro-damage, and bone turnover. In this review, we evaluated the impact of various pharmacological agents on osseointegration, from animal experiments conducting RTQ measurements. In addition to substances whose antiresorptive and/or anti-catabolic effects on bone are well-documented (e.g., alendronate, zoledronate, ibandronate, raloxifene, human parathyroid hormone, odanacatib, and the sclerostin monoclonal antibody), positive effects on RTQ have been reported for substances that do not primarily target bone (e.g., aminoguanidine, insulin, losartan, simvastatin, bone morphogenetic protein, alpha-tocopherol, and the combination of silk fibroin powder and platelet-rich fibrin). On the contrary, several substances (e.g., prednisolone, cyclosporin A, cisplatin, and enamel matrix derivative) tend to adversely impact RTQ. While morphometric parameters such as bone-implant contact appear to influence the biomechanical anchorage, increased or decreased RTQ is not always accompanied by corresponding fluctuations in bone-implant contact. This further confirms that factors such as bone quality underpin biomechanical anchorage of metal implants. Several fundamental questions on drug metabolism and bioavailability, drug dosage, animal-to-human translation, and the consequences of treatment interruption remain yet unanswered.
Keywords: Bisphosphonates; Bone; Drug delivery; Implant; In vivo; Osseointegration; Removal torque.
© 2022 The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

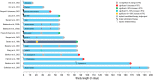
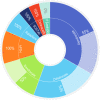
References
-
- Abtahi J., Agholme F., Sandberg O., Aspenberg P. Bisphosphonate-induced osteonecrosis of the jaw in a rat model arises first after the bone has become exposed. No primary necrosis in unexposed bone. J. Oral Pathol. Med. 2012;41:494–499. - PubMed
-
- Abtahi J., Agholme F., Sandberg O., Aspenberg P. Effect of local vs. systemic bisphosphonate delivery on dental implant fixation in a model of osteonecrosis of the jaw. J. Dent. Res. 2013;92:279–283. - PubMed
-
- Adell R., Lekholm U., Rockler B., Brånemark P.I. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int. J. Oral Surg. 1981;10:387–416. - PubMed
-
- Aerssens J., Boonen S., Lowet G., Dequeker J. Interspecies differences in bone composition, density, and quality: potential implications for in vivo bone research. Endocrinology. 1998;139:663–670. - PubMed
-
- Albrektsson T., Brånemark P.I., Hansson H.A., Lindström J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop. Scand. 1981;52:155–170. - PubMed
LinkOut - more resources
Full Text Sources

