An evaluation into the use of procalcitonin levels as a biomarker of bacterial sepsis to aid the management of intrapartum pyrexia and chorioamnionitis
- PMID: 36276783
- PMCID: PMC9563784
- DOI: 10.1016/j.xagr.2022.100064
An evaluation into the use of procalcitonin levels as a biomarker of bacterial sepsis to aid the management of intrapartum pyrexia and chorioamnionitis
Abstract
Background: Procalcitonin is an established biomarker for bacterial sepsis in the nonpregnant population with better diagnostic and prognostic value for bacterial infections.
Objective: This study aimed to evaluate whether procalcitonin levels could be used in the diagnosis and management of intrapartum sepsis in women and their neonates suspected of intrapartum bacterial sepsis.
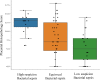
Study design: A prospective observational cohort study was conducted at the University Hospitals of Bristol and Weston NHS Foundation Trust. Overall, 117 women and their neonates managed for suspected intrapartum sepsis from June 2020 to October 2020 were included. Procalcitonin levels were measured in addition to routine biomarkers white cell count and C-reactive protein in women and their neonates during the initial septic screen and follow-up blood samples. The placentas underwent detailed histopathology. Maternal and neonatal parameters were used to categorize cases into "high-suspicion bacterial sepsis," "equivocal bacterial sepsis," and "low-suspicion bacterial sepsis." The Kruskal-Wallis test was used to compare categories with biomarker values and placental histology scores.
Results: Procalcitonin level was increased in 6 women in the initial septic screen sample, compared with 100 women with an increased C-reactive protein level. There was a significant difference in maternal postnatal procalcitonin results between "high-suspicion bacterial sepsis" and "low-suspicion bacterial sepsis" categories (P=.004). Moreover, 71.2% of placentas showed varying degrees of chorioamnionitis.
Conclusion: In our cohort of women, 94.6% had normal procalcitonin levels while in labor at the time of the septic screen, consistent with the low number of confirmed bacteremia. The result provided a basis that procalcitonin may complement clinical judgment and interpretation of already used prognostic and diagnostic tests, improving patient care in the management of intrapartum sepsis.
Keywords: chorioamnionitis; intrapartum sepsis; procalcitonin; sepsis biomarkers.
Crown Copyright © 2022 Published by Elsevier Inc.
Figures


References
-
- Greer O, Shah NM, Johnson MR. Maternal sepsis update: current management and controversies. Obstet Gynecol. 2020;22:45–55.
-
- Tujula B, Kokki H, Räsänen J, Kokki M. Procalcitonin; a feasible biomarker for severe bacterial infections in obstetrics and gynecology? Acta Obstet Gynecol Scand. 2018;97:505–506. - PubMed
-
- National Institute for Health and Care Excellence. Procalcitonin testing for diagnosing and monitoring sepsis (ADVIA Centaur BRAHMS PCT assay, BRAHMS PCT Sensitive Kryptor assay, Elecsys BRAHMS PCT assay, LIAISON BRAHMS PCT assay and VIDAS BRAHMS PCT assay). 2015. Available at: http://www.nice.org.uk/guidance/dg18. Accessed March 28, 2022.
-
- Agarwal R, Priyadarshini P, Mehndiratta M. Serum procalcitonin in pregnancy-associated sepsis: a case control study. S Afr J Obstet Gynaecol. 2019;25:15–19.
-
- Velasquez JESUS, Zuleta J, Portilla P, Caicedo K, Portilla L, Patiño J. 864: Usefulness of measuring procalcitonin (PTC) in pregnancy for the initial diagnosis of bacterial infection with systemic features. Am J Obstet Gynecol. 2018;218:S514–S515.
LinkOut - more resources
Full Text Sources
Research Materials

