Microcontact Printing of Biomolecules on Various Polymeric Substrates: Limitations and Applicability for Fluorescence Microscopy and Subcellular Micropatterning Assays
- PMID: 36277174
- PMCID: PMC9578008
- DOI: 10.1021/acsapm.2c00834
Microcontact Printing of Biomolecules on Various Polymeric Substrates: Limitations and Applicability for Fluorescence Microscopy and Subcellular Micropatterning Assays
Abstract
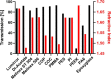
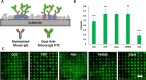
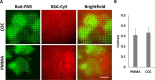
Polymeric materials play an emerging role in biosensing interfaces. Within this regard, polymers can serve as a superior surface for binding and printing of biomolecules. In this study, we characterized 11 different polymer foils [cyclic olefin polymer (COP), cyclic olefin copolymer (COC), polymethylmethacrylate (PMMA), DI-Acetate, Lumirror 4001, Melinex 506, Melinex ST 504, polyamide 6, polyethersulfone, polyether ether ketone, and polyimide] to test for the applicability for surface functionalization, biomolecule micropatterning, and fluorescence microscopy approaches. Pristine polymer foils were characterized via UV-vis spectroscopy. Functional groups were introduced by plasma activation and epoxysilane-coating. Polymer modification was evaluated by water contact angle measurement and X-ray photoelectron spectroscopy. Protein micropatterns were fabricated using microcontact printing. Functionalized substrates were characterized via fluorescence contrast measurements using epifluorescence and total internal reflection fluorescence microscopy. Results showed that all polymer substrates could be chemically modified with epoxide functional groups, as indicated by reduced water contact angles compared to untreated surfaces. However, transmission and refractive index measurements revealed differences in important optical parameters, which was further proved by fluorescence contrast measurements of printed biomolecules. COC, COP, and PMMA were identified as the most promising alternatives to commonly used glass coverslips, which also showed superior applicability in subcellular micropatterning experiments.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Soft lithography-based biomolecule patterning techniques and their applications in subcellular protein interaction analysis.Mater Today Bio. 2025 Mar 17;32:101672. doi: 10.1016/j.mtbio.2025.101672. eCollection 2025 Jun. Mater Today Bio. 2025. PMID: 40177382 Free PMC article. Review.
-
Fabrication, Characterization and Application of Biomolecule Micropatterns on Cyclic Olefin Polymer (COP) Surfaces with Adjustable Contrast.Biosensors (Basel). 2019 Dec 28;10(1):3. doi: 10.3390/bios10010003. Biosensors (Basel). 2019. PMID: 31905666 Free PMC article.
-
A Simplified and Robust Activation Procedure of Glass Surfaces for Printing Proteins and Subcellular Micropatterning Experiments.Biosensors (Basel). 2022 Feb 25;12(3):140. doi: 10.3390/bios12030140. Biosensors (Basel). 2022. PMID: 35323410 Free PMC article.
-
Facile immobilization of biomolecules onto various surfaces using epoxide-containing antibiofouling polymers.Langmuir. 2012 Mar 6;28(9):4507-14. doi: 10.1021/la204898y. Epub 2012 Feb 22. Langmuir. 2012. PMID: 22309129
-
Bio-functionalization of microfluidic platforms made of thermoplastic materials: A review.Anal Chim Acta. 2022 May 29;1209:339283. doi: 10.1016/j.aca.2021.339283. Epub 2021 Nov 22. Anal Chim Acta. 2022. PMID: 35569863 Review.
Cited by
-
High-throughput microcontact printing of proteins in microwell cell culture plates.MethodsX. 2024 Mar 12;12:102665. doi: 10.1016/j.mex.2024.102665. eCollection 2024 Jun. MethodsX. 2024. PMID: 38524307 Free PMC article.
-
Organic Electronics in Biosensing: A Promising Frontier for Medical and Environmental Applications.Biosensors (Basel). 2023 Nov 7;13(11):976. doi: 10.3390/bios13110976. Biosensors (Basel). 2023. PMID: 37998151 Free PMC article. Review.
-
Cell Patterning Technology on Polymethyl Methacrylate through Controlled Physicochemical and Biochemical Functionalization.Biosensors (Basel). 2023 Sep 23;13(10):904. doi: 10.3390/bios13100904. Biosensors (Basel). 2023. PMID: 37887097 Free PMC article.
-
Antibody-Based Array for Tacrolimus Immunosuppressant Monitoring with Planar Plastic Waveguides Activated with an Aminodextran-Lipase Conjugate.Anal Chem. 2024 Sep 3;96(35):14142-14149. doi: 10.1021/acs.analchem.4c02028. Epub 2024 Aug 22. Anal Chem. 2024. PMID: 39172628 Free PMC article.
-
Soft lithography-based biomolecule patterning techniques and their applications in subcellular protein interaction analysis.Mater Today Bio. 2025 Mar 17;32:101672. doi: 10.1016/j.mtbio.2025.101672. eCollection 2025 Jun. Mater Today Bio. 2025. PMID: 40177382 Free PMC article. Review.
References
-
- Salva M. L.; Rocca M.; Niemeyer C. M.; Delamarche E. Methods for Immobilizing Receptors in Microfluidic Devices: A Review. Micro Nano Eng. 2021, 11, 100085.10.1016/j.mne.2021.100085. - DOI
-
- Guruvenket S.; Rao G.; Komath M.; Raichur A. M. Plasma Surface Modification of Polystyrene and Polyethylene. Appl. Surf. Sci. 2004, 236, 278–284. 10.1016/j.apsusc.2004.04.033. - DOI
-
- Kim Y.-J.; Taniguchi Y.; Murase K.; Taguchi Y.; Sugimura H. Vacuum Ultraviolet-Induced Surface Modification of Cyclo-Olefin Polymer Substrates for Photochemical Activation Bonding. Appl. Surf. Sci. 2009, 255, 3648–3654. 10.1016/j.apsusc.2008.10.009. - DOI
LinkOut - more resources
Full Text Sources

