Store-operated calcium channels in skin
- PMID: 36277201
- PMCID: PMC9581152
- DOI: 10.3389/fphys.2022.1033528
Store-operated calcium channels in skin
Abstract
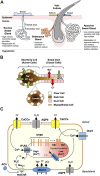
The skin is a complex organ that acts as a protective layer against the external environment. It protects the internal tissues from harmful agents, dehydration, ultraviolet radiation and physical injury as well as conferring thermoregulatory control, sensation, immunological surveillance and various biochemical functions. The diverse cell types that make up the skin include 1) keratinocytes, which form the bulk of the protective outer layer; 2) melanocytes, which protect the body from ultraviolet radiation by secreting the pigment melanin; and 3) cells that form the secretory appendages: eccrine and apocrine sweat glands, and the sebaceous gland. Emerging evidence suggests that store-operated Ca2+ entry (SOCE), whereby depletion of intracellular Ca2+ stores triggers Ca2+ influx across the plasma membrane, is central to the normal physiology of these cells and thus skin function. Numerous skin pathologies including dermatitis, anhidrotic ectodermal dysplasia, hyperhidrosis, hair loss and cancer are now linked to dysfunction in SOCE proteins. Principal amongst these are the stromal interaction molecules (STIMs) that sense Ca2+ depletion and Orai channels that mediate Ca2+ influx. In this review, the roles of STIM, Orai and other store-operated channels are discussed in the context of keratinocyte differentiation, melanogenesis, and eccrine sweat secretion. We explore not only STIM1-Orai1 as drivers of SOCE, but also independent actions of STIM, and emerging signal cascades stemming from their activities. Roles are discussed for the elusive transient receptor potential canonical channel (TRPC) complex in keratinocytes, Orai channels in Ca2+-cyclic AMP signal crosstalk in melanocytes, and Orai isoforms in eccrine sweat gland secretion.
Keywords: Orai channels; STIM; TRPC channels; eccrine sweat gland; keratinocytes; melanocytes; skin; store-operated calcium entry.
Copyright © 2022 Manning, Dart and Evans.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Bovell D. L., Robertson J., Evans R. (2016). STIM and Orai proteins regulate Ca2+ entry into isolated human eccrine sweat gland secretory coil cells. Proc. Physiol. Soc. 37, PCA137.
-
- Bovell D. L., Robertson J., Mohamed F. (2015). STIM and Orai proteins regulate calcium entry into human sweat gland cell line – NCL-SG3. Proc. Physiol. Soc. 34, PC050.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

