A facile synthesis of CeO2 from the GO@Ce-MOF precursor and its efficient performance in the oxygen evolution reaction
- PMID: 36277339
- PMCID: PMC9585184
- DOI: 10.3389/fchem.2022.996560
A facile synthesis of CeO2 from the GO@Ce-MOF precursor and its efficient performance in the oxygen evolution reaction
Abstract
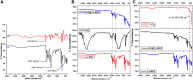
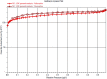
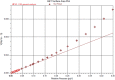
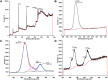
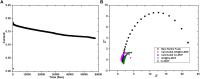
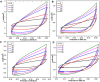
Electrochemical water splitting has enticed fascinating consideration as a key conduit for the advancement of renewable energy systems. Fabricating adequate electrocatalysts for water splitting is fervently preferred to curtail their overpotentials and hasten practical utilizations. In this work, a series of Ce-MOF, GO@Ce-MOF, calcinated Ce-MOF, and calcinated GO@Ce-MOF were synthesized and used as high-proficient electrocatalysts for the oxygen evolution reaction. The physicochemical characteristics of the prepared samples were measured by diverse analytical techniques including SEM, HRTEM, FTIR, BET, XPS, XRD, and EDX. All materials underwent cyclic voltammetry tests and were evaluated by electrochemical impedance spectroscopy and oxygen evolution reaction. Ce-MOF, GO@Ce-MOF, calcinated Ce-MOF, and calcinated GO@Ce-MOF have remarkable properties such as enhanced specific surface area, improved catalytic performance, and outstanding permanency in the alkaline solution (KOH). These factors upsurge ECSA and intensify the OER performance of the prepared materials. More exposed surface active-sites present in calcinated GO@Ce-MOF could be the logic for superior electrocatalytic activity. Chronoamperometry of the catalyst for 15°h divulges long-term stability of Ce-MOF during OER. Impedance measurements indicate higher conductivity of synthesized catalysts, facilitating the charge transfer reaction during electrochemical water splitting. This study will open up a new itinerary for conspiring highly ordered MOF-based surface active resources for distinct electrochemical energy applications.
Keywords: GO composites; MOF (metal–organic framework); cerium (3+); oxygen evolution reaction; water splitting.
Copyright © 2022 Ahmed Malik, Afaq, Mahmood, Niu, Yousaf ur Rehman, Ibrahim, Mohyuddin, Qureshi, Ashiq and Chughtai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Acar C., Dincer I. (2019). Review and evaluation of hydrogen production options for better environment. J. Clean. Prod. 218, 835–849. 10.1016/j.jclepro.2019.02.046 - DOI
-
- Almáši M., Zelenak V., Kuchar J., Bourrelly S., Llewellyn P. (2016). New members of MOF-76 family containing Ho (III) and Tm (III) ions: Characterization, stability and gas adsorption properties. Colloids Surfaces A Physicochem. Eng. Aspects 496, 114–124. 10.1016/j.colsurfa.2015.10.048 - DOI
-
- Almáši M., Zelenak V., Opanasenko M., Cisarova I. (2015). Ce (III) and Lu (III) metal–organic frameworks with Lewis acid metal sites: Preparation, sorption properties and catalytic activity in Knoevenagel condensation. Catal. Today 243, 184–194. 10.1016/j.cattod.2014.07.028 - DOI
-
- Amaro-Gahete J., Klee R., Esquivel D., Ruiz J. R., Jimenez-Sanchidrian C., Romero-Salguero F. J. (2019). Fast ultrasound-assisted synthesis of highly crystalline MIL-88A particles and their application as ethylene adsorbents. Ultrason. Sonochem. 50, 59–66. 10.1016/j.ultsonch.2018.08.027 - DOI - PubMed
LinkOut - more resources
Full Text Sources

