Relation between CarS expression and activation of carotenogenesis by stress in Fusarium fujikuroi
- PMID: 36277400
- PMCID: PMC9581392
- DOI: 10.3389/fbioe.2022.1000129
Relation between CarS expression and activation of carotenogenesis by stress in Fusarium fujikuroi
Abstract
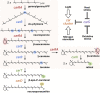
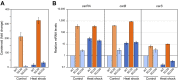
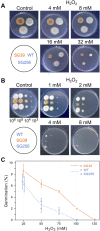
Fusarium fujikuroi, a model organism for secondary metabolism in fungi, produces carotenoids, terpenoid pigments with antioxidant activity. Previous results indicate that carotenoid synthesis in F. fujikuroi is stimulated by light or by different stress conditions and downregulated by a RING finger protein encoded by carS gene. Here, we have analyzed the effects of three stressors, nitrogen scarcity, heat shock, and oxidative stress. We compared them with the effect of light in the wild type, a carS mutant that overproduces carotenoids, and its complemented strain. The assayed stressors increase the synthesis of carotenoids in the three strains, but mRNA levels of structural genes of carotenogenesis, carRA and carB, are only enhanced in the presence of a functional carS gene. In the wild-type strain, the four conditions affect in different manners the mRNA levels of carS: greater in the presence of light, without significant changes in nitrogen starvation, and with patent decreases after heat shock or oxidative stress, suggesting different activation mechanisms. The spores of the carS mutant are more resistant to H2O2 than those of the wild type; however, the mutant shows a greater H2O2 sensitivity at the growth level, which may be due to the participation of CarS in the regulation of genes with catalase domains, formerly described. A possible mechanism of regulation by heat stress has been found in the alternative splicing of the intron of the carS gene, located close to its 3' end, giving rise to the formation of a shorter protein. This action could explain the inducing effect of the heat shock, but not of the other inducing conditions, which may involve other mechanisms of action on the CarS regulator, either transcriptionally or post-transcriptionally.
Keywords: CarS; alternative splicing; heat shock; intron; light; nitrogen starvation; oxidative stress; photoinduction.
Copyright © 2022 Ruger-Herreros, Nordzieke, Vega-Álvarez, Avalos and Limón.
Conflict of interest statement
SN is employed by the Symrise AG Chemicals company. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
LinkOut - more resources
Full Text Sources

