Nanomaterials in diagnostics, imaging and delivery: Applications from COVID-19 to cancer
- PMID: 36277435
- PMCID: PMC9576318
- DOI: 10.1557/s43579-022-00257-7
Nanomaterials in diagnostics, imaging and delivery: Applications from COVID-19 to cancer
Abstract
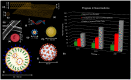
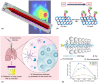
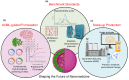
Abstract: In the past two decades, the emergence of nanomaterials for biomedical applications has shown tremendous promise for changing the paradigm of all aspects of disease management. Nanomaterials are particularly attractive for being a modularly tunable system; with the ability to add functionality for early diagnostics, drug delivery, therapy, treatment and monitoring of patient response. In this review, a survey of the landscape of different classes of nanomaterials being developed for applications in diagnostics and imaging, as well as for the delivery of prophylactic vaccines and therapeutics such as small molecules and biologic drugs is undertaken; with a particular focus on COVID-19 diagnostics and vaccination. Work involving bio-templated nanomaterials for high-resolution imaging applications for early cancer detection, as well as for optimal cancer treatment efficacy, is discussed. The main challenges which need to be overcome from the standpoint of effective delivery and mitigating toxicity concerns are investigated. Subsequently, a section is included with resources for researchers and practitioners in nanomedicine, to help tailor their designs and formulations from a clinical perspective. Finally, three key areas for researchers to focus on are highlighted; to accelerate the development and clinical translation of these nanomaterials, thereby unleashing the true potential of nanomedicine in healthcare.
Keywords: Biological; Biomedical; COVID-19; Nanoscale; Nanostructure.
© The Author(s) 2022.
Conflict of interest statement
Conflict of interestThe authors declare that they have no conflict of interest.
Figures



Similar articles
-
Inorganic nanomaterials with rapid clearance for biomedical applications.Chem Soc Rev. 2021 Aug 7;50(15):8669-8742. doi: 10.1039/d0cs00461h. Epub 2021 Jun 22. Chem Soc Rev. 2021. PMID: 34156040 Review.
-
Self-Assembled Organic Nanomaterials for Drug Delivery, Bioimaging, and Cancer Therapy.ACS Biomater Sci Eng. 2020 Sep 14;6(9):4816-4833. doi: 10.1021/acsbiomaterials.0c00883. Epub 2020 Aug 13. ACS Biomater Sci Eng. 2020. PMID: 33455214 Review.
-
Engineered Hybrid Nanoparticles for On-Demand Diagnostics and Therapeutics.Acc Chem Res. 2015 Dec 15;48(12):3016-25. doi: 10.1021/acs.accounts.5b00316. Epub 2015 Nov 25. Acc Chem Res. 2015. PMID: 26605438
-
Biomedical applications of gold nanomaterials: opportunities and challenges.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015 Nov-Dec;7(6):779-96. doi: 10.1002/wnan.1341. Epub 2015 Mar 24. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015. PMID: 25808787 Review.
-
Biobased Nanomaterials: Pioneering Innovations for Biomedical Advancements.Pharm Nanotechnol. 2024 Mar 18. doi: 10.2174/0122117385291530240305044703. Online ahead of print. Pharm Nanotechnol. 2024. PMID: 38504570
Cited by
-
Nano Revolution: "Tiny tech, big impact: How nanotechnology is driving SDGs progress".Heliyon. 2024 May 16;10(10):e31393. doi: 10.1016/j.heliyon.2024.e31393. eCollection 2024 May 30. Heliyon. 2024. PMID: 38818162 Free PMC article. Review.
-
Nanomedicine and clinical diagnostics part I: applications in conventional imaging (MRI, X-ray/CT, and ultrasound).Nanomedicine (Lond). 2025 Jan;20(2):167-182. doi: 10.1080/17435889.2024.2439776. Epub 2024 Dec 11. Nanomedicine (Lond). 2025. PMID: 39661327 Review.
-
Nanobiomaterials & nanomedicine.J Transl Med. 2024 Dec 27;22(1):1154. doi: 10.1186/s12967-024-06005-w. J Transl Med. 2024. PMID: 39731150 Free PMC article. No abstract available.
-
Poly(β-amino ester)s-based nanovehicles: Structural regulation and gene delivery.Mol Ther Nucleic Acids. 2023 Apr 23;32:568-581. doi: 10.1016/j.omtn.2023.04.019. eCollection 2023 Jun 13. Mol Ther Nucleic Acids. 2023. PMID: 37200860 Free PMC article. Review.
-
Instance maps as an organising concept for complex experimental workflows as demonstrated for (nano)material safety research.Beilstein J Nanotechnol. 2025 Jan 22;16:57-77. doi: 10.3762/bjnano.16.7. eCollection 2025. Beilstein J Nanotechnol. 2025. PMID: 39877837 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
