Evaluation of Short-Term Symptoms Associated With COVID-19 Vaccines Used Among Adolescents in Saudi Arabia
- PMID: 36277554
- PMCID: PMC9580612
- DOI: 10.7759/cureus.29306
Evaluation of Short-Term Symptoms Associated With COVID-19 Vaccines Used Among Adolescents in Saudi Arabia
Abstract
Objectives: Several government-sponsored reporting systems have stated mild to moderate side effects of COVID-19 vaccines. However, patient-reported data on COVID-19 vaccine-associated adverse effects in adolescents are lacking. Our objective was to assess the short-term side effects of Pfizer-BioNTech BNT162b2 or Moderna mRNA-1273 vaccinations among teenagers in Saudi Arabia.
Methods: A retrospective, cross-sectional study was conducted among individuals aged 12-18 years old who received one of the two mentioned vaccines between July 2021 and March 2022 in Riyadh, Saudi Arabia.
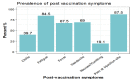
Results: The most common short-term side effects reported for COVID-19 vaccines among teenagers in our study were fatigue, pain at the site of injection, fever, chills, headache, nausea, and vomiting. Female participants, individuals who had a history of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, and those who received two doses of the vaccine are at higher risk to develop side effects after getting the vaccine. Importantly, asthmatic participants have a higher incidence of COVID-19 vaccine side effects when compared to those with no history of chronic diseases.
Conclusion: Our findings might enhance public trust in the COVID-19 vaccine, which could speed up the immunization procedure.
Keywords: covid-19; moderna mrna-1273 vaccine; pfizer-biontech vaccine; side effects; teenagers.
Copyright © 2022, Alrowdhan et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines.Eur Rev Med Pharmacol Sci. 2021 Feb;25(3):1663-1669. doi: 10.26355/eurrev_202102_24877. Eur Rev Med Pharmacol Sci. 2021. PMID: 33629336 Review.
-
Side Effects of COVID-19 Pfizer-BioNTech mRNA Vaccine in Children Aged 12-18 Years in Saudi Arabia.Vaccines (Basel). 2021 Nov 9;9(11):1297. doi: 10.3390/vaccines9111297. Vaccines (Basel). 2021. PMID: 34835228 Free PMC article.
-
Safety Profile of COVID-19 Vaccines among Healthcare Workers in Poland.Vaccines (Basel). 2022 Mar 12;10(3):434. doi: 10.3390/vaccines10030434. Vaccines (Basel). 2022. PMID: 35335066 Free PMC article.
-
COVID-19 Vaccines Breakthrough Infections and Adverse Effects Reported by the Birzeit University Community in Palestine.Int J Gen Med. 2024 Jul 30;17:3349-3360. doi: 10.2147/IJGM.S466838. eCollection 2024. Int J Gen Med. 2024. PMID: 39100722 Free PMC article.
-
Pfizer-BioNTech COVID-19 Vaccine (BNT162b2) Side Effects: A Systematic Review.Cureus. 2022 Mar 26;14(3):e23526. doi: 10.7759/cureus.23526. eCollection 2022 Mar. Cureus. 2022. PMID: 35494952 Free PMC article. Review.
References
-
- Silver Spring, MD: US Department of Health and Human Services, Food and Drug Administration; [ May; 2022 ]. 2020. FDA: Pfizer-BioNTech COVID-19 vaccine emergency use authorization review memorandum. Silver Spring, MD: US Department of Health and Human Services, Food and Drug Administration.
-
- Food and Drug Administration. and. Silver Spring, MD: US Department of Health and Human Services, Food and Drug Administration; [ May; 2022 ]. 2021. FDA: Pfizer-BioNTech COVID-19 vaccine EUA amendment review memorandum. Silver Spring, MD: US Department of Health and Human Services; p. 2021.
-
- Weaver J. Weaver J: Moderna files for FDA authorization for Covid vaccine in younger teens, adolescents. NBC News. [ Feb; 2022 ]. 2021. https://www.nbcnews.com/health/kids-health/moderna-files-authorization-c... https://www.nbcnews.com/health/kids-health/moderna-files-authorization-c...
LinkOut - more resources
Full Text Sources
Miscellaneous