Intramolecular tetrazine-acryloyl cycloaddition: chemistry and applications
- PMID: 36277625
- PMCID: PMC9473534
- DOI: 10.1039/d2sc04331a
Intramolecular tetrazine-acryloyl cycloaddition: chemistry and applications
Abstract
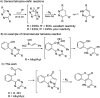
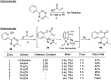
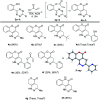
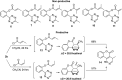
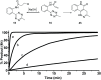
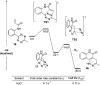
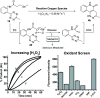
An unprecedented intramolecular [4 + 2] tetrazine-olefin cycloaddition with α,β-unsaturated substrates was discovered. The reaction produces unique coumarin-dihydropyridazine heterocycles that exhibited strong fluorescence with large Stokes shifts and excellent photo- and pH-stability. This property can be used for reaction analysis. The rate of cycloaddition was found to be solvent dependent and was determined using experimental data with a kinetic modeling software (COPASI) as well as DFT calculations (k 1 = 0.64 ± 0.019 s-1 and 4.1 s-1, respectively). The effects of steric and electronic properties of both the tetrazine and α,β-unsaturated carbonyl on the reaction were studied and followed the known trends characteristic of the intermolecular reaction. Based on these results, we developed a "release-then-click" strategy for the ROS triggered release of methylselenenic acid (MeSeOH) and a fluorescent tracer. This strategy was demonstrated in HeLa cells via fluorescence imaging.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

References
LinkOut - more resources
Full Text Sources

