Evolution of the active species of homogeneous Ru hydrodeoxygenation catalysts in ionic liquids
- PMID: 36277633
- PMCID: PMC9473539
- DOI: 10.1039/d2sc02150a
Evolution of the active species of homogeneous Ru hydrodeoxygenation catalysts in ionic liquids
Abstract
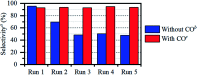
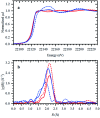
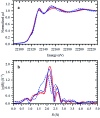
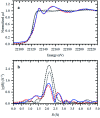
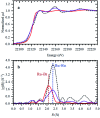
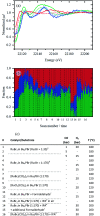
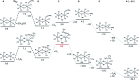
This work establishes structure-property relationships in Ru-based catalytic systems for selective hydrodeoxygenation of ketones to alkenes by combining extensive catalytic testing, in situ X-ray absorption spectroscopy (XAS) under high pressures and temperatures and ex situ XAS structural characterization supported by density functional theory (DFT) calculations. Catalytic tests revealed the difference in hydrogenation selectivity for ketones (exemplified by acetone) or alkenes (exemplified by propene) upon changing the reaction conditions, more specifically in the presence of CO during a pretreatment step. XAS data demonstrated the evolution of the local ruthenium structure with different amounts of Cl/Br and CO ligands. In addition, in the absence of CO, the catalyst was reduced to Ru0, and this was associated with a significant decrease of the selectivity for ketone hydrogenation. For the Ru-bromide carbonyl complex, selectivity towards acetone hydrogenation over propene hydrogenation was explained on the basis of different relative energies of the first intermediate states of each reaction. These results give a complete understanding of the evolution of the Ru species, used for the catalytic valorization of biobased polyols to olefins in ionic liquids, identifying the undesired deactivation routes as well as possibilities for reactivation.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


References
-
- Dub P. A. Gordon J. C. Nat. Rev. Chem. 2018;2:396–408. doi: 10.1038/s41570-018-0049-z. - DOI
-
- Evans D. Osborn J. A. Jardine F. H. Wilkinson G. Nature. 1965;208:1203–1204. doi: 10.1038/2081203b0. - DOI
-
- Ohkuma T. Ooka H. Hashiguchi S. Ikariya T. Noyori R. J. Am. Chem. Soc. 1995;117:2675–2676. doi: 10.1021/ja00114a043. - DOI
-
- Braca G. Raspolli Galletti A. M. Sbrana G. J. Organomet. Chem. 1991;417:41–49. doi: 10.1016/0022-328X(91)80159-H. - DOI
LinkOut - more resources
Full Text Sources

