Stability matters, too - the thermodynamics of amyloid fibril formation
- PMID: 36277637
- PMCID: PMC9473512
- DOI: 10.1039/d1sc06782f
Stability matters, too - the thermodynamics of amyloid fibril formation
Abstract
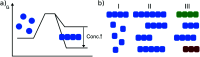
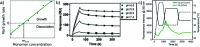
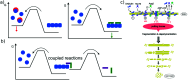
Amyloid fibrils are supramolecular homopolymers of proteins that play important roles in biological functions and disease. These objects have received an exponential increase in attention during the last few decades, due to their role in the aetiology of a range of severe disorders, most notably some of a neurodegenerative nature. While an overwhelming number of experimental studies exist that investigate how, and how fast, amyloid fibrils form and how their formation can be inhibited, a much more limited body of experimental work attempts to answer the question as to why these types of structures form (i.e. the thermodynamic driving force) and how stable they actually are. In this review, I attempt to give an overview of the types of experiments that have been performed to-date to answer these questions, and to summarise our current understanding of amyloid thermodynamics.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




Similar articles
-
Thermodynamics of amyloid fibril formation from non-equilibrium experiments of growth and dissociation.Biophys Chem. 2021 Apr;271:106549. doi: 10.1016/j.bpc.2021.106549. Epub 2021 Jan 29. Biophys Chem. 2021. PMID: 33578107
-
The growth of amyloid fibrils: rates and mechanisms.Biochem J. 2019 Oct 15;476(19):2677-2703. doi: 10.1042/BCJ20160868. Biochem J. 2019. PMID: 31654060 Review.
-
Thermodynamics of amyloid fibril formation from chemical depolymerization.Phys Chem Chem Phys. 2019 Dec 4;21(47):26184-26194. doi: 10.1039/c9cp04524d. Phys Chem Chem Phys. 2019. PMID: 31755512
-
Insights into Stabilizing Forces in Amyloid Fibrils of Differing Sizes from Polarizable Molecular Dynamics Simulations.J Mol Biol. 2018 Oct 12;430(20):3819-3834. doi: 10.1016/j.jmb.2018.05.020. Epub 2018 May 18. J Mol Biol. 2018. PMID: 29782833
-
Understanding amyloid fibril nucleation and aβ oligomer/drug interactions from computer simulations.Acc Chem Res. 2014 Feb 18;47(2):603-11. doi: 10.1021/ar4002075. Epub 2013 Dec 24. Acc Chem Res. 2014. PMID: 24368046 Review.
Cited by
-
Energy Landscapes and Heat Capacity Signatures for Monomers and Dimers of Amyloid-Forming Hexapeptides.Int J Mol Sci. 2023 Jun 25;24(13):10613. doi: 10.3390/ijms241310613. Int J Mol Sci. 2023. PMID: 37445791 Free PMC article.
-
Catalytically Active Amyloids as Future Bionanomaterials.Nanomaterials (Basel). 2022 Oct 28;12(21):3802. doi: 10.3390/nano12213802. Nanomaterials (Basel). 2022. PMID: 36364578 Free PMC article. Review.
-
Simulations of Amyloid-Forming Peptides in the Crystal State.Protein J. 2023 Jun;42(3):192-204. doi: 10.1007/s10930-023-10119-3. Epub 2023 May 5. Protein J. 2023. PMID: 37145206 Free PMC article.
-
Martini on the Rocks: Can a Coarse-Grained Force Field Model Crystals?J Phys Chem Lett. 2024 Feb 1;15(4):1079-1088. doi: 10.1021/acs.jpclett.4c00012. Epub 2024 Jan 23. J Phys Chem Lett. 2024. PMID: 38261634 Free PMC article.
-
Self-Assembly of Polymers and Their Applications in the Fields of Biomedicine and Materials.Polymers (Basel). 2024 Jul 23;16(15):2097. doi: 10.3390/polym16152097. Polymers (Basel). 2024. PMID: 39125124 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

