Three's a crowd - stabilisation, structure, and applications of DNA triplexes
- PMID: 36277639
- PMCID: PMC9473520
- DOI: 10.1039/d2sc01793h
Three's a crowd - stabilisation, structure, and applications of DNA triplexes
Abstract
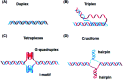
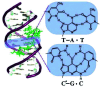
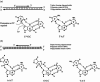
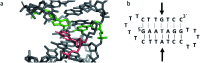
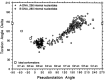
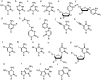
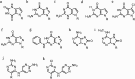
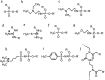
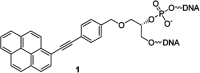
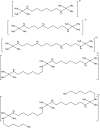
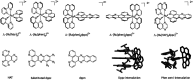
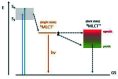
DNA is a strikingly flexible molecule and can form a variety of secondary structures, including the triple helix, which is the subject of this review. The DNA triplex may be formed naturally, during homologous recombination, or can be formed by the introduction of a synthetic triplex forming oligonucleotide (TFO) to a DNA duplex. As the TFO will bind to the duplex with sequence specificity, there is significant interest in developing TFOs with potential therapeutic applications, including using TFOs as a delivery mechanism for compounds able to modify or damage DNA. However, to combine triplexes with functionalised compounds, a full understanding of triplex structure and chemical modification strategies, which may increase triplex stability or in vivo degradation, is essential - these areas will be discussed in this review. Ruthenium polypyridyl complexes, which are able to photooxidise DNA and act as luminescent DNA probes, may serve as a suitable photophysical payload for a TFO system and the developments in this area in the context of DNA triplexes will also be reviewed.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
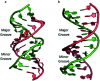


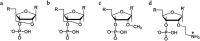






Similar articles
-
Triplex DNA structures.Annu Rev Biochem. 1995;64:65-95. doi: 10.1146/annurev.bi.64.070195.000433. Annu Rev Biochem. 1995. PMID: 7574496 Review.
-
A new approach to overcome potassium-mediated inhibition of triplex formation.Nucleic Acids Res. 1996 Oct 1;24(19):3858-65. doi: 10.1093/nar/24.19.3858. Nucleic Acids Res. 1996. PMID: 8871568 Free PMC article.
-
Investigation of the formation and intracellular stability of purine.(purine/pyrimidine) triplexes.Nucleic Acids Res. 1997 May 15;25(10):1965-74. doi: 10.1093/nar/25.10.1965. Nucleic Acids Res. 1997. PMID: 9115364 Free PMC article.
-
Promotion of duplex and triplex DNA formation by polycation comb-type copolymers.Methods Mol Med. 2001;65:209-24. doi: 10.1385/1-59259-139-6:209. Methods Mol Med. 2001. PMID: 21318757
-
DNA triple helices: biological consequences and therapeutic potential.Biochimie. 2008 Aug;90(8):1117-30. doi: 10.1016/j.biochi.2008.02.011. Epub 2008 Feb 21. Biochimie. 2008. PMID: 18331847 Free PMC article. Review.
Cited by
-
Interactions of arene ruthenium(II) complexes [η6-(C6H6)Ru(pprip)Cl]+ and [η6-(C6H6)Ru(H2iiP)Cl]+ with RNA triplex poly(U)•poly(A)*poly(U).J Biol Inorg Chem. 2023 Sep;28(6):559-570. doi: 10.1007/s00775-023-02008-y. Epub 2023 Jul 21. J Biol Inorg Chem. 2023. PMID: 37477757
-
Interactions of small molecules with DNA junctions.Nucleic Acids Res. 2022 Dec 9;50(22):12636-12656. doi: 10.1093/nar/gkac1043. Nucleic Acids Res. 2022. PMID: 36382400 Free PMC article. Review.
-
Binding and stabilizating effect of RNA triplex poly(U)⋅poly(A)*poly(U) by enantiomers of ruthenium(II) polypyridyl complex [Ru(bpy)2(dppx)]2.J Biol Inorg Chem. 2023 Aug;28(5):509-517. doi: 10.1007/s00775-023-02004-2. Epub 2023 Jul 15. J Biol Inorg Chem. 2023. PMID: 37452869
-
Switching on Supramolecular DNA Junction Binding Using a Human Enzyme.Angew Chem Int Ed Engl. 2025 May;64(21):e202503683. doi: 10.1002/anie.202503683. Epub 2025 Mar 23. Angew Chem Int Ed Engl. 2025. PMID: 40088149 Free PMC article.
-
Advancing Topoisomerase Research Using DNA Nanotechnology.Small Methods. 2025 Jun;9(6):e2401113. doi: 10.1002/smtd.202401113. Epub 2024 Nov 11. Small Methods. 2025. PMID: 39526512 Free PMC article. Review.
References
-
- Alberts B., Johnson A., Lewis J., Raff M., Roberts K. and Walter P., in Molecular Biology of the Cell, Garland Science, 4th edn., 2002
-
- Voet D. and Voet J. G., Biochemistry, Pedersen, New York, 2nd edn, 1995, vol. 1
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

