Antibacterial Ti-Cu implants: A critical review on mechanisms of action
- PMID: 36278144
- PMCID: PMC9579810
- DOI: 10.1016/j.mtbio.2022.100447
Antibacterial Ti-Cu implants: A critical review on mechanisms of action
Abstract
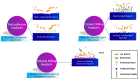
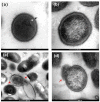
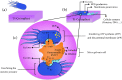
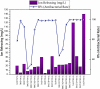
Titanium (Ti) has been widely used for manufacturing of bone implants because of its mechanical properties, biological compatibility, and favorable corrosion resistance in biological environments. However, Ti implants are prone to infection (peri-implantitis) by bacteria which in extreme cases necessitate painful and costly revision surgeries. An emerging, viable solution for this problem is to use copper (Cu) as an antibacterial agent in the alloying system of Ti. The addition of copper provides excellent antibacterial activities, but the underpinning mechanisms are still obscure. This review sheds light on such mechanisms and reviews how incorporation of Cu can render Ti-Cu implants with antibacterial activity. The review first discusses the fundamentals of interactions between bacteria and implanted surfaces followed by an overview of the most common engineering strategies utilized to endow an implant with antibacterial activity. The underlying mechanisms for antibacterial activity of Ti-Cu implants are then discussed in detail. Special attention is paid to contact killing mechanisms because the misinterpretation of this mechanism is the root of discrepancies in the literature.
Keywords: Anti-infection; Antibacterial mechanisms; Antimicrobial; Biomaterials; Contact killing; Ion releasing; Ti–Cu implants.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures









References
-
- Gristina A.G. Biomaterial-centered infection: microbial adhesion versus tissue integration. Science. 1987;237(4822):1588–1595. - PubMed
-
- Arciola C.R., Campoccia D., Montanaro L. Implant infections: adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018;16(7):397–409. - PubMed
-
- Rasouli M.R., Restrepo C., Maltenfort M.G., Purtill J.J., Parvizi J. Risk factors for surgical site infection following total joint arthroplasty. J Bone Joint Surg Am. 2014;96(18):e158. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

