Calycosin attenuates renal ischemia/reperfusion injury by suppressing NF-κB mediated inflammation via PPARγ/EGR1 pathway
- PMID: 36278223
- PMCID: PMC9585199
- DOI: 10.3389/fphar.2022.970616
Calycosin attenuates renal ischemia/reperfusion injury by suppressing NF-κB mediated inflammation via PPARγ/EGR1 pathway
Abstract
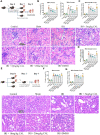
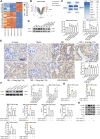
Renal ischemia reperfusion injury (IRI) is a leading and common cause of acute kidney injury (AKI), and inflammation is a critical factor in ischemic AKI progression. Calycosin (CAL), a major active component of Radix astragali, has been reported to have anti-inflammatory effect in multiple organs. However, whether CAL can alleviate renal IRI and its mechanism remain uncertain. In the present study, a renal IRI model is established by bilateral renal pedicles occlusion for 35 min in male C57BL/6 mice, and the effect of CAL on renal IRI is measured by serum creatinine and pathohistological assay. Hypoxia/reoxygenation (H/R) stimulated human renal tubular epithelial cells HK-2 were applied to explore the regulatory mechanisms of CAL. Luciferase reporter assay and molecular docking were applied to identify the CAL's target protein and pathway. In the mice with renal IRI, CAL dose dependently alleviated the renal injury and decreased nuclear factor kappa B (NF-κB) mediated inflammatory response. Bioinformatics analysis and experiments showed that early growth response 1 (EGR1) increased in mice with renal IRI and promoted NF-κB mediated inflammatory processes, and CAL dose-dependably reduced EGR1. Through JASPAR database and luciferase reporter assay, peroxisome proliferator-activated receptor γ (PPARγ) was predicted to be a transcription factor of EGR1 and repressed the expression of EGR1 in renal tubular epithelial cells. CAL could increase PPARγ in a dose dependent manner in mice with renal IRI and molecular docking predicted CAL could bind stably to PPARγ. In HK-2 cells after H/R, CAL increased PPARγ, decreased EGR1, and inhibited NF-κB mediated inflammatory response. However, PPARγ knockdown by siRNA transfection abrogated the anti-inflammation therapeutic effect of CAL. CAL produced a protective effect on renal IRI by attenuating NF-κB mediated inflammatory response via PPARγ/EGR1 pathway.
Keywords: acute kidney injury; calycosin; early growth response 1; ischemia/reperfusion injury; peroxisome proliferator-activated receptor γ.
Copyright © 2022 Zhang, Guan, Liu, Li, Yang, Xu, Niu, Zhao, Zhou, Che, Wang and Xu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials

