It is all About the Chase: Neurosteroidogenesis in Male Rats is Driven by Control of Mating Pace
- PMID: 36278466
- PMCID: PMC10472806
- DOI: 10.2174/1570159X21666221019114535
It is all About the Chase: Neurosteroidogenesis in Male Rats is Driven by Control of Mating Pace
Abstract
Background: Masculine sexual behaviors are dependent on androstane-derived steroids; however, the modulatory effects of mating, and of mating control, on androstane neurosteroidogenesis remain largely unknown.
Objective: Herein, we investigated the effects of mating control, prior sexual experience, and age on brain region specific neurosteroidogenic responses in male rats.
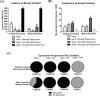
Methods: Effects of acute sexual experience were tested in naïve male rats that either remained sexually- naïve, were exposed to a standard mating chamber, or were either given control of the mating pace in a standard mating chamber (male control) or mated wherein the female stimulus rat controlled the mating pace in a paced-mating chamber (female control). Aged (10-12 months) sexually responsive male rats were similarly euthanized from the homecage or engaged in male controlled or female controlled mating. All rats were euthanized immediately following exposure conditions for radioimmunoassay of steroids in midbrain, hypothalamus, hippocampus and cortex.
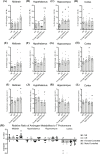
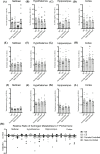
Results: Consummatory sexual behavior in male vs. female-controlled mating paradigms was altered by age and prior sexual experience. Male-controlled mating increased androstane neurosteroid metabolism, such that complementary increases in the testosterone (T) metabolite 5α-androstane-3α-17β- diol (3α-diol) in the midbrain and hypothalamus of male rats corresponded to decreases in the prohormone, T. 3α-diol were increased in the hippocampus in response to the context alone, and to a lesser degree in response to mating. Mating diminished neurosteroidogenesis in the cortex. Neurosteroidogenesis was overall reduced in aged male rats compared to naïve controls, however, these effects were more prominent in sexually non-responsive aged male rats.
Conclusion: Extending previous findings, these results indicate differential production of androstane neurosteroids in a mating exposure, age and brain region dependent manner.
Keywords: 5α-androstane-3α-17β-diol (3α-diol); Mating; aging; estradiol; paced-mating; testosterone.
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Conflict of interest statement
The authors declare no conflict of interest, financial or otherwise.
Figures

Similar articles
-
Mating stimuli influence endogenous variations in the neurosteroids 3alpha,5alpha-THP and 3alpha-Diol.J Neuroendocrinol. 1999 Nov;11(11):839-47. doi: 10.1046/j.1365-2826.1999.00379.x. J Neuroendocrinol. 1999. PMID: 10520134
-
Learning and the Lifespan: What's Sex Got to Do With It?Front Neurosci. 2020 Mar 20;14:216. doi: 10.3389/fnins.2020.00216. eCollection 2020. Front Neurosci. 2020. PMID: 32265631 Free PMC article.
-
Effects of non-contingent cocaine on 3alpha-androstanediol. I. Disruption of male sexual behavior.Physiol Behav. 2019 May 1;203:120-127. doi: 10.1016/j.physbeh.2017.12.017. Epub 2017 Dec 14. Physiol Behav. 2019. PMID: 29248633
-
The role of neurosteroids and non-genomic effects of progestins and androgens in mediating sexual receptivity of rodents.Brain Res Brain Res Rev. 2001 Nov;37(1-3):201-22. doi: 10.1016/s0165-0173(01)00119-9. Brain Res Brain Res Rev. 2001. PMID: 11744087 Review.
-
The testosterone metabolite and neurosteroid 3alpha-androstanediol may mediate the effects of testosterone on conditioned place preference.Brain Res Brain Res Rev. 2001 Nov;37(1-3):162-71. doi: 10.1016/s0165-0173(01)00116-3. Brain Res Brain Res Rev. 2001. PMID: 11744084 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

