Fexinidazole for Human African Trypanosomiasis, the Fruit of a Successful Public-Private Partnership
- PMID: 36278589
- PMCID: PMC9589988
- DOI: 10.3390/diseases10040090
Fexinidazole for Human African Trypanosomiasis, the Fruit of a Successful Public-Private Partnership
Abstract
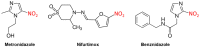
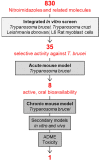
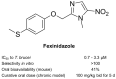
After 100 years of chemotherapy with impractical and toxic drugs, an oral cure for human African trypanosomiasis (HAT) is available: Fexinidazole. In this case, we review the history of drug discovery for HAT with special emphasis on the discovery, pre-clinical development, and operational challenges of the clinical trials of fexinidazole. The screening of the Drugs for Neglected Diseases initiative (DNDi) HAT-library by the Swiss TPH had singled out fexinidazole, originally developed by Hoechst (now Sanofi), as the most promising of a series of over 800 nitroimidazoles and related molecules. In cell culture, fexinidazole has an IC50 of around 1 µM against Trypanosoma brucei and is more than 100-fold less toxic to mammalian cells. In the mouse model, fexinidazole cures both the first, haemolymphatic, and the second, meningoencephalitic stage of the infection, the latter at 100 mg/kg twice daily for 5 days. In patients, the clinical trials managed by DNDi and supported by Swiss TPH mainly conducted in the Democratic Republic of the Congo demonstrated that oral fexinidazole is safe and effective for use against first- and early second-stage sleeping sickness. Based on the positive opinion issued by the European Medicines Agency in 2018, the WHO has released new interim guidelines for the treatment of HAT including fexinidazole as the new therapy for first-stage and non-severe second-stage sleeping sickness caused by Trypanosoma brucei gambiense (gHAT). This greatly facilitates the diagnosis and treatment algorithm for gHAT, increasing the attainable coverage and paving the way towards the envisaged goal of zero transmission by 2030.
Keywords: Trypanosoma brucei; clinical trial efficiency; clinical trial operations; clinical trial planning; drug discovery; human African trypanosomiasis; nitroimidazole; product development partnership; sleeping sickness.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

