Yeast Surface Display Platform for Rapid Selection of an Antibody Library via Sequential Counter Antigen Flow Cytometry
- PMID: 36278614
- PMCID: PMC9590018
- DOI: 10.3390/antib11040061
Yeast Surface Display Platform for Rapid Selection of an Antibody Library via Sequential Counter Antigen Flow Cytometry
Abstract
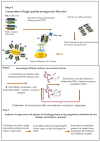
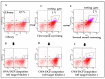
Yeast surface display techniques have been increasingly employed as a tool for both the discovery and affinity maturation of antibodies. In this study, we describe the use of yeast surface display for the selection and affinity maturation of antibodies targeted to small molecules (haptens). In this approach, we coupled 4 to 15 sequential cycles of error-prone PCR to introduce heterogeneity into the sequence of an 12F6 scFv antibody that binds to chelated uranium; the resulting full-length constructs were combined to create a yeast-displayed scFv-library with high diversity. We also developed a stringent selection technique utilizing fluorescence-activated cell sorting; this was based on sequentially dropping the target antigen concentration, while concomitantly increasing the concentration of potential cross-reactive haptens in subsequent selection cycles. As a proof of the efficacy this approach, we confirmed that the antibodies identified via this approach retained binding to the target antigen (UO22+ complexed to a chelator), while binding with lesser affinity than the parental scFv to a structurally related haptens (the same chelator complexed to other metal ions). As will be described in this report, these scFv variants perform more efficiently in sensor-based assay than the parental 12F6 antibody. Combining the generation of scFv libraries via error-prone PCR with selection of yeast-displayed antibodies by fluorescence activated cell sorting will provide an efficient new method for the isolation of scFvs and other binding proteins with high affinity and specificity.
Keywords: affinity maturation; antibody-based biosensors; equilibrium binding constants; error prone PCR; flow cytometry; hexavalent uranium (UO22+); single chain variable fragments (scFv); yeast surface display.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publications of this paper.
Figures





Similar articles
-
Rapid Affinity Maturation of Novel Anti-PD-L1 Antibodies by a Fast Drop of the Antigen Concentration and FACS Selection of Yeast Libraries.Biomed Res Int. 2019 Dec 28;2019:6051870. doi: 10.1155/2019/6051870. eCollection 2019. Biomed Res Int. 2019. PMID: 31976323 Free PMC article.
-
Yeast Surface Display in Combination with Fluorescence-activated Cell Sorting Enables the Rapid Isolation of Antibody Fragments Derived from Immunized Chickens.Biotechnol J. 2019 Apr;14(4):e1800466. doi: 10.1002/biot.201800466. Epub 2018 Nov 28. Biotechnol J. 2019. PMID: 30350923
-
Affinity maturation of antibodies by combinatorial codon mutagenesis versus error-prone PCR.MAbs. 2020 Jan-Dec;12(1):1803646. doi: 10.1080/19420862.2020.1803646. MAbs. 2020. PMID: 32744131 Free PMC article.
-
Construction and screening of antigen targeted immune yeast surface display antibody libraries.Curr Protoc Cytom. 2008 Jul;Chapter 4:Unit4.7. doi: 10.1002/0471142956.cy0407s45. Curr Protoc Cytom. 2008. PMID: 18770649 Review.
-
Development of Recombinant Antibody by Yeast Surface Display Technology.Curr Res Pharmacol Drug Discov. 2024 Jan 20;6:100174. doi: 10.1016/j.crphar.2024.100174. eCollection 2024. Curr Res Pharmacol Drug Discov. 2024. PMID: 38318280 Free PMC article. Review.
Cited by
-
Agglutination of yeast-binding antibodies from human blood plasma products.BMC Microbiol. 2025 Jul 29;25(1):457. doi: 10.1186/s12866-025-03989-3. BMC Microbiol. 2025. PMID: 40730944 Free PMC article.
-
Matrix metalloproteinase-driven epithelial-mesenchymal transition: implications in health and disease.J Transl Med. 2025 Apr 11;23(1):436. doi: 10.1186/s12967-025-06447-w. J Transl Med. 2025. PMID: 40217300 Free PMC article. Review.
-
Quantifying antibody binding: techniques and therapeutic implications.MAbs. 2025 Dec;17(1):2459795. doi: 10.1080/19420862.2025.2459795. Epub 2025 Feb 16. MAbs. 2025. PMID: 39957177 Free PMC article. Review.
References
-
- Kearns-Jonker M., Barteneva N., Mencel R., Hussain N., Shulkin I., Xu A., Yew M., Cramer D.V. Use of Molecular Modeling and Site-Directed Mutagenesis to Define the Structural Basis for the Immune Response to Carbohydrate Xenoantigens. BMC Immunol. 2007;8:3. doi: 10.1186/1471-2172-8-3. - DOI - PMC - PubMed
-
- McCullum E.O., Williams B.A.R., Zhang J., Chaput J.C. Random Mutagenesis by Error-Prone PCR. Methods Mol. Biol. Clifton NJ. 2010;634:103–109. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

