A Simple Method for the Prediction of Therapeutic Proteins (Monoclonal and Polyclonal Antibodies and Non-Antibody Proteins) for First-in-Pediatric Dose Selection: Application of Salisbury Rule
- PMID: 36278619
- PMCID: PMC9590058
- DOI: 10.3390/antib11040066
A Simple Method for the Prediction of Therapeutic Proteins (Monoclonal and Polyclonal Antibodies and Non-Antibody Proteins) for First-in-Pediatric Dose Selection: Application of Salisbury Rule
Abstract
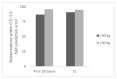
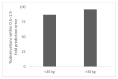
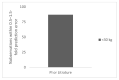
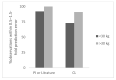
In order to conduct a pediatric clinical trial, it is important to optimize pediatric dose as accurately as possible. In this study, a simple weight-based method known as ‘Salisbury Rule’ was used to predict pediatric dose for therapeutic proteins and was then compared with the observed pediatric dose. The observed dose was obtained mainly from the FDA package insert and if dosing information was not available from the FDA package insert then the observed dose was based on the dose given to an age group in a particular study. It was noted that the recommended doses of most of the therapeutic proteins were extrapolated to pediatrics from adult dose based on per kilogram (kg) body weight basis. Since it is widely believed that pediatric dose should be selected based on the pediatric clearance (CL), a CL based pediatric dose was projected from the following equation: Dose in children = Adult dose × (Observed CL in children/Observed adult CL). In this study, this dose was also considered observed pediatric dose for comparison. A ±30% prediction error (predicted vs. observed) was considered acceptable. There were 21 monoclonal antibodies, 5 polyclonal antibodies in children ≥ 2 years of age, 4 polyclonal antibodies in preterm and term neonates, and 11 therapeutic proteins (non-antibodies) in the study. In children < 30 kg body weight, the predicted doses were within 0.5−1.5-fold prediction error for 87% (monoclonal antibody), 100% (polyclonal antibody), and 92% (non-antibodies) observations. In children > 30 kg body weight, the predicted doses were within 0.5−1.5-fold prediction error for 96% (monoclonal antibody), 100% (polyclonal antibody), and 100% (non-antibodies) observations. The Salisbury Rule mimics more to CL-based dose rather than per kg body weight-based extrapolated dose from adults. The Salisbury Rule for the pediatric dose prediction can be used to select first-in-children dose in pediatric clinical trials and may be in clinical settings.
Keywords: Salisbury Rule; body weight; clearance; pediatric dose.
Conflict of interest statement
The author declares no conflict of interest.
Figures
Similar articles
-
A comparison of different methods for the first-in-pediatric dose selection.J Clin Transl Res. 2022 Sep 7;8(5):369-381. eCollection 2022 Oct 31. J Clin Transl Res. 2022. PMID: 36518546 Free PMC article.
-
Application of Allometric Scaling and Salisbury Rule for the Prediction of Antimalarial Drugs for First-in-Pediatric Dose Selection.Eur J Drug Metab Pharmacokinet. 2023 Sep;48(5):587-594. doi: 10.1007/s13318-023-00848-2. Epub 2023 Aug 11. Eur J Drug Metab Pharmacokinet. 2023. PMID: 37566210
-
Prediction of Clearance of Monoclonal and Polyclonal Antibodies and Non-Antibody Proteins in Children: Application of Allometric Scaling.Antibodies (Basel). 2020 Aug 5;9(3):40. doi: 10.3390/antib9030040. Antibodies (Basel). 2020. PMID: 32764408 Free PMC article.
-
Pediatric Dose Selection for Therapeutic Proteins.J Clin Pharmacol. 2021 Jun;61 Suppl 1:S193-S206. doi: 10.1002/jcph.1829. J Clin Pharmacol. 2021. PMID: 34185910 Review.
-
Pediatric physiology in relation to the pharmacokinetics of monoclonal antibodies.Expert Opin Drug Metab Toxicol. 2018 Jun;14(6):585-599. doi: 10.1080/17425255.2018.1482278. Epub 2018 Jun 4. Expert Opin Drug Metab Toxicol. 2018. PMID: 29806953 Review.
Cited by
-
Opportunities and challenges of pharmacovigilance in special populations: a narrative review of the literature.Ther Adv Drug Saf. 2023 Sep 28;14:20420986231200746. doi: 10.1177/20420986231200746. eCollection 2023. Ther Adv Drug Saf. 2023. PMID: 37780667 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

