Advanced glycation end products induce senescence of atrial myocytes and increase susceptibility of atrial fibrillation in diabetic mice
- PMID: 36278684
- PMCID: PMC9741501
- DOI: 10.1111/acel.13734
Advanced glycation end products induce senescence of atrial myocytes and increase susceptibility of atrial fibrillation in diabetic mice
Abstract
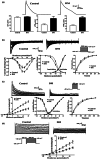
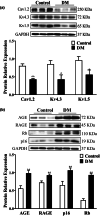
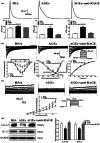
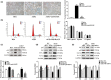
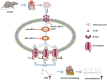
Diabetes mellitus (DM) is a common chronic metabolic disease caused by significant accumulation of advanced glycation end products (AGEs). Atrial fibrillation (AF) is a common cardiovascular complication of DM. Here, we aim to clarify the role and mechanism of atrial myocyte senescence in the susceptibility of AF in diabetes. Rapid transesophageal atrial pacing was used to monitor the susceptibility of mice to AF. Whole-cell patch-clamp was employed to record the action potential (AP) and ion channels in single HL-1 cell and mouse atrial myocytes. More importantly, anti-RAGE antibody and RAGE-siRNA AAV9 were used to investigate the relationship among diabetes, aging, and AF. The results showed that elevated levels of p16 and retinoblastoma (Rb) protein in the atrium were associated with increased susceptibility to AF in diabetic mice. Mechanistically, AGEs increased p16/Rb protein expression and the number of SA-β-gal-positive cells, prolonged the action potential duration (APD), reduced protein levels of Cav1.2, Kv1.5, and current density of ICa,L , IKur in HL-1 cells. Anti-RAGE antibody or RAGE-siRNA AAV9 reversed these effects in vitro and in vivo, respectively. Furthermore, downregulating p16 or Rb by siRNA prevented AGEs-mediated reduction of Cav1.2 and Kv1.5 proteins expression. In conclusion, AGEs accelerated atrial electrical remodeling and cellular senescence, contributing to increased AF susceptibility by activating the p16/Rb pathway. Inhibition of RAGE or the p16/Rb pathway may be a potential therapeutic target for AF in diabetes.
Keywords: AGEs; atrial fibrillation; cell senescence; diabetes; electrical remodeling; p16 and Rb.
© 2022 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no conflict of interest. All institutional and national guidelines for the care and use of laboratory animals were followed.
Figures

References
-
- Alam, P. , Haile, B. , Arif, M. , Pandey, R. , Rokvic, M. , Nieman, M. , Maliken, B. D. , Paul, A. , Wang, Y. G. , Sadayappan, S. , Ahmed, R. P. H. , & Kanisicak, O. (2019). Inhibition of Senescence‐Associated Genes Rb1 and Meis2 in Adult Cardiomyocytes Results in Cell Cycle Reentry and Cardiac Repair Post‐Myocardial Infarction. Journal of the American Heart Association, 8, e012089. 10.1161/jaha.119.012089 - DOI - PMC - PubMed
-
- Baker, D. J. , Childs, B. G. , Durik, M. , Wijers, M. E. , Sieben, C. J. , Zhong, J. , Saltness, R. A. , Jeganathan, K. B. , Verzosa, G. C. , Pezeshki, A. , Khazaie, K. , Miller, J. D. , & van Deursen, J. M. (2016). Naturally occurring p16(Ink4a)‐positive cells shorten healthy lifespan. Nature, 530, 184–189. 10.1038/nature16932 - DOI - PMC - PubMed
-
- Barana, A. , Matamoros, M. , Dolz‐Gaitón, P. , Pérez‐Hernández, M. , Amorós, I. , Núñez, M. , Sacristán, S. , Pedraz, Á. , Pinto, Á. , Fernández‐Avilés, F. , Tamargo, J. , Delpón, E. , & Caballero, R. (2014). Chronic atrial fibrillation increases microRNA‐21 in human atrial myocytes decreasing L‐type calcium current. Circulation: Arrhythmia and Electrophysiology, 7, 861–868. 10.1161/circep.114.001709 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

