Recalibrating vision-for-action requires years after sight restoration from congenital cataracts
- PMID: 36278872
- PMCID: PMC9633067
- DOI: 10.7554/eLife.78734
Recalibrating vision-for-action requires years after sight restoration from congenital cataracts
Abstract
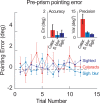
Being able to perform adept goal-directed actions requires predictive, feed-forward control, including a mapping between the visually estimated target locations and the motor commands reaching for them. When the mapping is perturbed, e.g., due to muscle fatigue or optical distortions, we are quickly able to recalibrate the sensorimotor system to update this mapping. Here, we investigated whether early visual and visuomotor experience is essential for developing sensorimotor recalibration. To this end, we assessed young individuals deprived of pattern vision due to dense congenital bilateral cataracts who were surgically treated for sight restoration only years after birth. We compared their recalibration performance to such distortion to that of age-matched sighted controls. Their sensorimotor recalibration performance was impaired right after surgery. This finding cannot be explained by their still lower visual acuity alone, since blurring vision in controls to a matching degree did not lead to comparable behavior. Nevertheless, the recalibration ability of cataract-treated participants gradually improved with time after surgery. Thus, the lack of early pattern vision affects visuomotor recalibration. However, this ability is not lost but slowly develops after sight restoration, highlighting the importance of sensorimotor experience gained late in life.
Keywords: congenital cataracts; human; neuroscience; prism adaptation; sensorimotor development; sight recovery; visuomotor recalibration.
© 2022, Senna et al.
Conflict of interest statement
IS, SP, IB, ME No competing interests declared
Figures






Similar articles
-
The power of vision: calibration of auditory space after sight restoration from congenital cataracts.Proc Biol Sci. 2022 Oct 12;289(1984):20220768. doi: 10.1098/rspb.2022.0768. Epub 2022 Oct 5. Proc Biol Sci. 2022. PMID: 36196538 Free PMC article.
-
Development of multisensory integration following prolonged early-onset visual deprivation.Curr Biol. 2021 Nov 8;31(21):4879-4885.e6. doi: 10.1016/j.cub.2021.08.060. Epub 2021 Sep 16. Curr Biol. 2021. PMID: 34534443
-
Sight restoration in congenitally blind humans does not restore visual brain structure.Cereb Cortex. 2023 Feb 20;33(5):2152-2161. doi: 10.1093/cercor/bhac197. Cereb Cortex. 2023. PMID: 35580850
-
Diagnosis and management of cataracts in infancy and childhood.Ophthalmic Surg. 1984 Aug;15(8):688-97. Ophthalmic Surg. 1984. PMID: 6384869 Review.
-
Cataracts in children.J Cataract Refract Surg. 2005 Apr;31(4):824-40. doi: 10.1016/j.jcrs.2005.01.012. J Cataract Refract Surg. 2005. PMID: 15899463 Review.
Cited by
-
Changes in effective connectivity during the visual-motor integration tasks: a preliminary f-NIRS study.Behav Brain Funct. 2024 Mar 11;20(1):4. doi: 10.1186/s12993-024-00232-3. Behav Brain Funct. 2024. PMID: 38468270 Free PMC article.
-
Multisensory training improves the development of spatial cognition after sight restoration from congenital cataracts.iScience. 2024 Feb 9;27(3):109167. doi: 10.1016/j.isci.2024.109167. eCollection 2024 Mar 15. iScience. 2024. PMID: 38414862 Free PMC article.
-
Visual experience shapes the Bouba-Kiki effect and the size-weight illusion upon sight restoration from congenital blindness.Sci Rep. 2023 Jul 15;13(1):11435. doi: 10.1038/s41598-023-38486-y. Sci Rep. 2023. PMID: 37454205 Free PMC article.
-
Postural control depends on early visual experience.J Vis. 2024 Sep 3;24(9):3. doi: 10.1167/jov.24.9.3. J Vis. 2024. PMID: 39226067 Free PMC article.
-
Postural stability and optic flow sensitivity following sight restoration from congenital bilateral cataracts.Proc Biol Sci. 2025 Jun;292(2048):20250787. doi: 10.1098/rspb.2025.0787. Epub 2025 Jun 4. Proc Biol Sci. 2025. PMID: 40461067
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

